Denzel
Modelling Using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
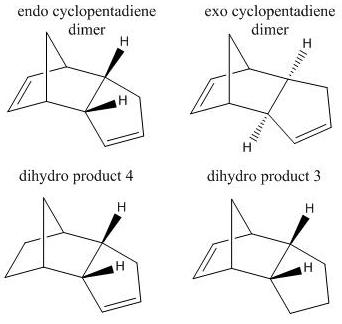
Cyclopentadiene monomer units can react to form dimer units via a [4+2] diels-alder cycloaddition. Given the diels-alder mechanism, the dimer products can be classified as either endo or exo. By use of a molecular modelling program such as Chem-Bio 3D, it is possible to determine which cyclopentadiene dimer - endo or exo - is the kinetic/thermodynamic product. With reference to the table below and analysis of data corresponding to the energies of the exo/endo-cyclopentadiene products, it is clear to see that endo-cyclopentadiene is the kinetic product and the thermodynamic product is the exo-cyclopentadiene. This is due to exo-cyclopentadiene displaying the lowest conformational energy, thus indicating the most thermodynamically stable dimer. However it is also noted that dimerisation results in the endocyclopentadiene being the major product, thus the reaction is kinetcially controlled.
Endo-cyclopentadiene dimer can be reduced forming two Dihydro derivatives. Given that there are two alkene functionalities present for hydrogenation, further analysis using an MM2 approxiamtion in Chem-Bio 3D can be used to calculate the respective enrgies of the reduced products. From this it can be noted that Dihydro product 4 is the thermodynamic product of hydrogenation. Also the table illustrates that energy contributions from stretches (5.36 and 4.60 kJ/mol) is small in relation to bending energies (83.26 and 60.63 kJ/mol), indicating the molecule is more bent relative to normality. Small energy contributions from hyrogen bonding/(dipole/dipole) (0.67 and 0.56 kJ/mol) are experienced but are small due to the lack of an electronegative atom.
| Energy Contribution | Exo-Cyclopentadiene Dimer | Endo-Cyclopentadiene Dimer | Dihydro Derivative 3 | Dihydro Derivative 4 |
|---|---|---|---|---|
| Stretch | 5.40 | 5.23 | 5.36 | 4.60 |
| Bend | 86.15 | 87.28 | 83.26 | 60.63 |
| Stretch-Bend | -3.51 | -3.47 | -3.51 | -2.30 |
| Torsion | 32.09 | 39.79 | 45.10 | 52.34 |
| 1,4 Van Der waals | 17.70 | 17.99 | 23.56 | 18.87 |
| Non-1,4 Van Der Waals | -5.98 | -6.40 | -5.15 | -4.35 |
| Dipole/Dipole | 1.59 | 1.88 | 0.67 | 0.56 |
| Total Energy (kJ/mol) | 133.43 | 142.00 | 149.24 | 130.42 |
Stereochemistry of Nucleophillic Additions to a Pyridinium Ring (NAD+ analogue)
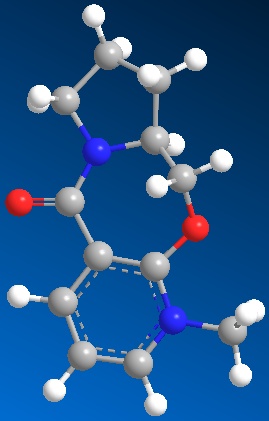
Grignards reagents are commonly used for alkylation reactions, the reaction proceeds via nucleophillic attack of a carboanion. Below shows the addition of methyl to a pyridinium ring, whereby the grignards selectively attacks the 4 position of the alpha-beta-unsaturated carbonyl functionality. Using Chem-Bio3D it was possible to minimise the conformational energy of the starting material (reactant), MMFF94 force field was used. The resulting structure exhibited an overall minimum energy of 240.45 kJ/mol.
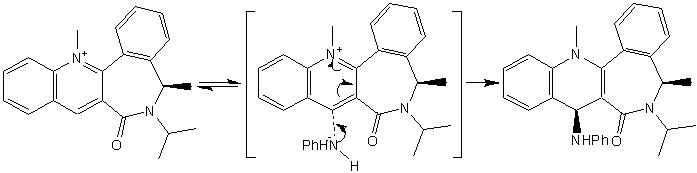
As the mechanism shows, Magnesium datively binds to the oxygen molecule present on the carbonyl, this determines the highly selctive stereochemsitry of the alkylated product.
A similar reaction was studied using Chem-Bio3D, whereby NHPh was added to a pyridine ring. MMFF94 was again used to optimise the energy of the reactant, this was found to be 411.57 kJ/mol. By observation of the molecule at this energy, it is evident that there are steric issues that will influence the overall stereochemistry of the product.
This is due to the non-planar carbonyl function in relation to the pyridine ring. Subsequently the addition of the NHPh is such that interactions are minimal, in this case NHPh positions itself away from the carbonyl function. Thus the reaction is controlled through steric hinderence rather than co-ordination of reactants.
More accurate dtermination of the lowest energy conformation could be obtained by using the MOPAC/PM6 approximation, this is a more realistic representaion of the energy as it considers the influence of molecular orbital interactions.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
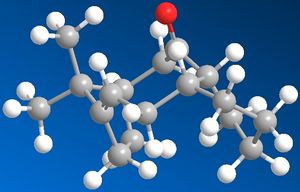

Atropisomers 9 and 10 were proposed by Paquette as the intermediates in the synthesis of Taxol. Atropisomeriastion has arisen in this intermediate molecule since there are large steric effects, namely the bicyclic conformation, that do not enable the free roation of the sigma bonds connecting the carbonyl functionality. Thus the molecule can be categorised as either having the carbonyl facing up (9) or having the carbonyl facing down (10).
By inspection of molecule 9, it is clear to see that there is transannular strain present due to the steric and electronic clash of the carbonyl with the similarly orientated hydrogen atoms. This is energetically unfavourable, and concequently increases the energy of conformation of the intermediate. Also the cyclohaxane ring of 9 adopts an energetically unfavourable twist boat conformation, again contributing to the increased enrgy of the intermediate. Chembio3D and molecular modelling approximation MM2 was used to determine the lowest energy of this intermediate, it was found to be 227.55 kJ/mol.
Similar analysis of molecule 10 indicates the absence of the large transannular strain, since the carbonyl group is pointing away from the hydrogen atoms, thereby reducing any unfavourable steric or electronic interactions. The energetically favourable chair conformation is also adopted by the cyclohexane ring, contributing to the lowering in the total conformational energy of the intermediate molecule 10. Chembio3D and molecular modelling approximation MM2 was used to determine the lowest energy of this intermediate, it was found to be 185.41 kJ/mol.
Bridgehead olefins are preferred in medium-sized polycyclic molecules, and are categorised as hyperstable olefins [1] . They exhibit negative olefin strain, such that the strain energy of the bridgehead olefin is less than that of its parent hydrocarbon. This is a thermodynaic driving force of formation of the polycyclic intermediate molecule.
Hyperstable olefin theory also provides reasoning for the unusually slow reactivity of the olefin functionality within the intermediate molecules 9 and 10.
Modelling Using Semi-Empirical Molecular Orbital Theory
Previous molecular mechanical approximations can be improved by the use of semi-empirical methods, whereby conformational energies for molecules are obtained with consideration of atomic orbital interactions. This model can be used to relate classical mechanics to quantum mechanical theories.
Regioselective Addition of Dichlorocarbene
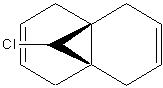
When minimisng the conformational energy of molecule 12, MM2 force-field was used (74.97 kJ/mol) prior to subjecting the molecule to a MOPAC/PM6 MO semi-empirical calculation. This lead to a graphical representation of the valence electron wavefunctions, and noticably changed the conformation of the molecule with respect to the MM2 force field calculations.
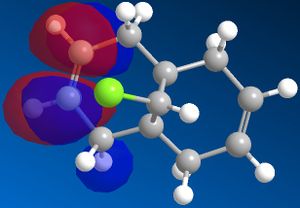
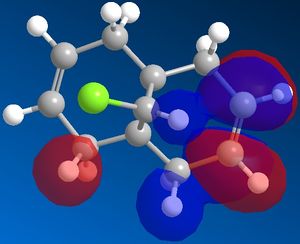
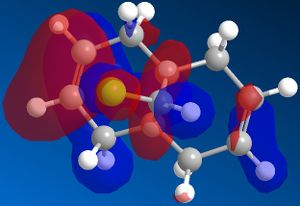
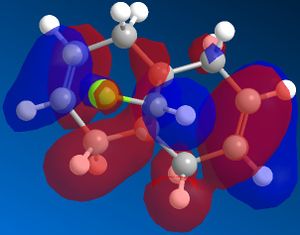
Different molecular orbitals could be illustrated using MOPAC/PM6 programme, the 'HOMO-1' upto the 'LUMO+2' molecular orbitals are shown below. By use of these approximations for the wavefunctions, it was opossible to investiagte into the reactivity of the molecule with electrophillic reagents such as dichlorocarbene.
Reactions involving electrophillic reagents require the HOMO wavefunction to be studied. There are two possible olefin sites availible for electrophillic attack, however by observation of the graphical representations of the HOMO it is evident that the there is likely to be highly selective electrophillic attack of the alkene bond that is syn to the chlorine atom. This is due to the large excess of pi electron density surroundinig the syn olefin in relation the the anti olefin.
 |
 |
 |
 |
 |
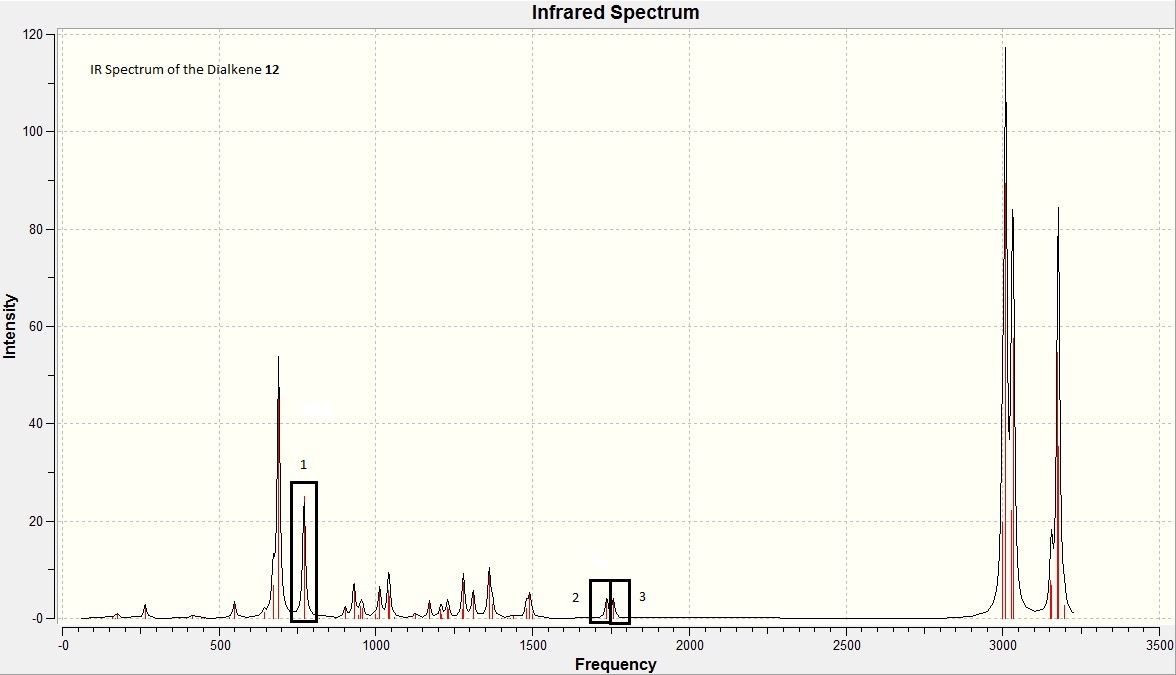
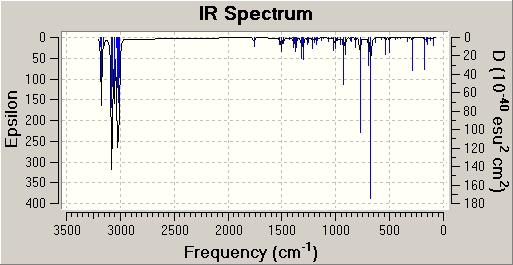
To investigate the influence of the chlorine heteroatom upon the vibrational frequencies of the alkene bonds, the diene and a monohydrogenated derivative (exo sigma bond) were subjected to B3LYP/6-31G(d,p) Gaussian geometry optimization and frequency calculations. Prior to optimisation by Gaussian, the aforementioned techniques were performed - (MM2 force field and MOPAC/PM6).
Spectra obtained was as follows: (diene - left)/(monoene - right)
|
|
| Bond type | Monoene Frequency (/cm) | Diene frequency (/cm) | Lit. Frequency (/cm) |
|---|---|---|---|
| C=C (syn) | 1758.07 | 1757.35 | 1620-1680 |
| C=C (anti) | 1737.03 | 1620-1680 | |
| C-Cl | 774.98 | 770.88 | 600-800 |
From the table of frequencies, it can be noted that the symmetrical stretching frequency for the syn C=C bond is higher than that for the anti C=C bond. This indicates that the syn alkene is stronger than the anti alkene, thus suggesting the anti alkene will be more reactive than the syn alkene.
This outcome contradicts the previously suggested reactivity of the diene based upon the Gaussian MO calculations.
It can also be noted that the literature values for the C=C symmetrical stretch is far lower than that observed experimentally, this is likely to be due to stabilisation of the C=C by the highly electronegative Chlorine atom.
Structure based Mini project using DFT-based Molecular orbital methods
Assigning regioisomers in "Click Chemistry"
Azides react with alkynes in a [3+2] cycloaddition reaction producing 1,2,3-triazoles. This reaction can be catalysed by two types of metal, each producing different dominant isomers - Ruthenium (II) produces the 1,5-isomer and Copper (I) produces the 1,4-isomer.
The reaction kinetics are very rapid when catalysed with copper (I) and is known as the click reaction, which is widely used to generate tailored substances quickly and reliably by joining small units together.
In this project, the forementioned methods for energy minimisation - MM2 force field, MOPAC/PM6 and Gaussian DFT - will be combined to determine an approxiation for the 13C NMR for the two isomers. These could then be compared with that of literature[4].
13C NMR Analysis
The following are the NMR spectra approxiamtions obtained by Gaussview calculations: (left - Isomer 1)/(right - isomer 2)
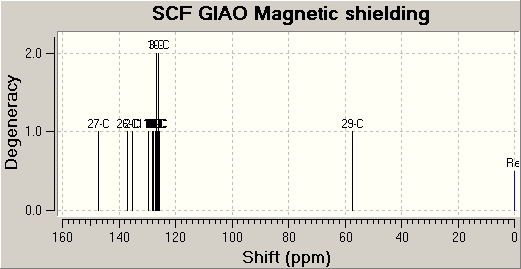
|
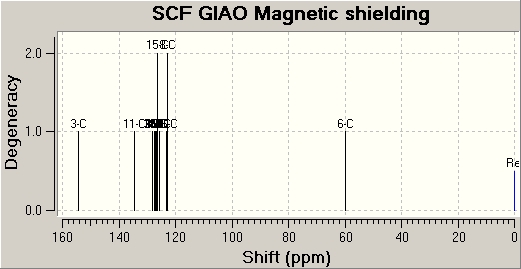
|
Literature values [5] for the 13C NMR was as follows: 13C NMR (CDCl3): δ 51.85, 126.93, 127.22, 128.22, 128.92, 129.08, 129.64, 133.26, 133.34, 135.66, 138.26.
| Carbon Number | Isomer 1 (ppm) | Isomer 2 (ppm) |
|---|---|---|
| 1 | 137.16 | 127.05 |
| 2 | 147.38 | 154.41 |
| 3 | 125.71 | 128.26 |
| 4 | 128.23 | 123.02 |
| 5 | 126.76 | 126.70 |
| 6 | 127.72 | 125.52 |
| 7 | 125.99 | 127.24 |
| 8 | 129.64 | 123.02 |
| 9 | 57.49 | 59.78 |
| 10 | 135.34 | 134.60 |
| 11 | 126.87 | 126.29 |
| 12 | 126.28 | 126.83 |
| 13 | 125.99 | 126.29 |
| 14 | 127.18 | 127.39 |
| 15 | 126.87 | 126.47 |
By comparison of the literature values with that of the experiemental Gaussview approximations for the 13C NMR, it can be deduced that the molecule in literature is that of isomer 2, as is expected when catalysed by Ruthenium (II).
Examination of steric influences within the two isomers indicates that isomer 1 does not appear as energetically favourable as isomer 2, this suggests the Copper metal catalysis follows a kinetic pathway, and thus the Ruthenium metal cataysis follows the thermodynamic pathway. Explaining the difference in kinetics between the two catalysed reactions.
Another method of determination of the isomers would be to examine melting points, since van-der waals intermolecular forces will be greately different in the two isomers and thus melting point will differ. Such that Isomer 1 will have a lower melting point than isomer 2.
References and Citations
- ↑ Hyperstable Olefins: Further Calculational Explorations and Predictions: {(DOI|10.1021/ja00274a016)}
- ↑ Characteristic IR Absorption Frequencies of Organic Functional Groups{(DOI|http://www2.ups.edu/faculty/hanson/Spectroscopy/IR/IRfrequencies.html)}
- ↑ Typical Infrared Absorption Frequencies{(DOI|http://www.cem.msu.edu/~reusch/VirtualText/Spectrpy/InfraRed/infrared.htm)}
- ↑ Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides{(DOI|10.1021/ja054114s)}
- ↑ Supporting Information for Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides{(DOI|http://pubs.acs.org/doi/suppl/10.1021/ja054114s/suppl_file/ja054114ssi20051014_012328.pdf)}