Ct1515MRD
Exercise 1: H + H2
Question 1
What value does the total gradient of the potential energy surface have at a minimum and at a transition structure? Briefly explain how minima and transition structures can be distinguished using the curvature of the potential energy surface.
At the minimum point the total gradient of potential energy is 0 and at the transition state (maxima) it will also be 0. They can be distinguished by taking the second derivative and the minima value will a postive value where as the maxima (transition state) value will give a negative second derivative.
(This won't be true if you take the second derivative along the axis where the transition state sits at a minimum. Lt912 (talk) 03:38, 2 June 2017 (BST))
Question 2 - Location of Transition state
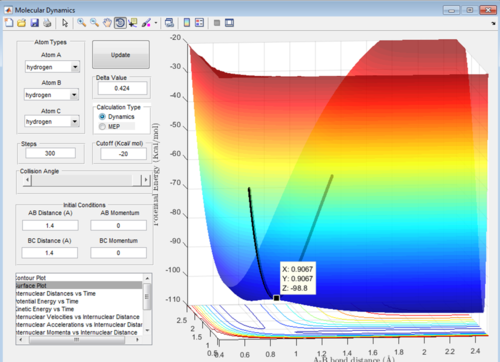
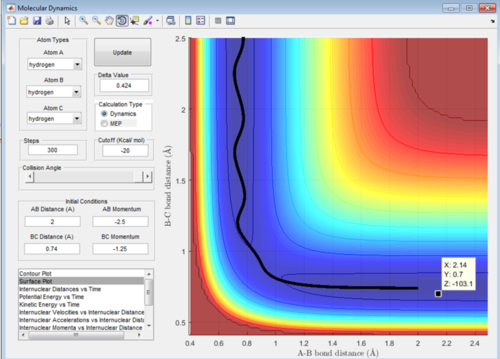
To find the transition state initially conditions with r1 = r2 = 1.4, and p1 = p2 = 0.0. An initial estimate of the transition state was found to be X= 0.9067 Å, Y= 0.9067 Å and Z= -98.8 Kcal/mol. This can be seen in Figure 1 and was found by roughly estimating the maxima value by using the curser.
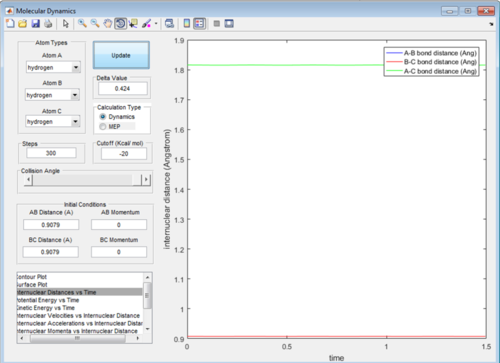
Upon adjustment of the internuclear distance when the bond length was 0.9079 Å the fluctuations of bond length became negligible. This can be seen in Figure 2 and shows that the trasition state bond lenth had been achieved.
Question 3 - Calculating the reaction path
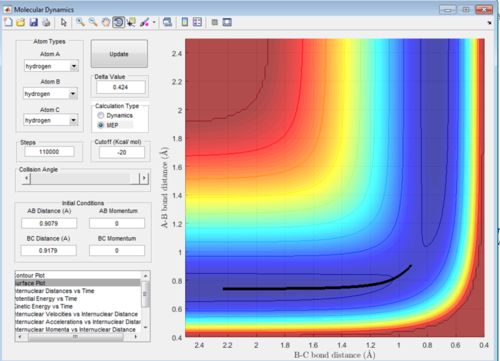
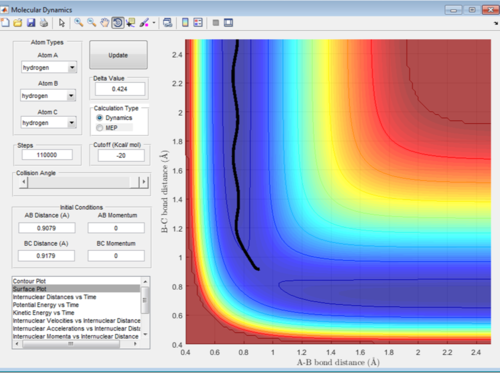
Comment on how the mep and the trajectory you just calculated differ.
Figure 3 and 4 show the reaction pathway with MEP analysis and Dynamics analysis respectively. A small change of 0.01 was made to the value of r2. As is can be seen the MEP analysis doesn't take into account the velocity and shows a very straight trajectory. Dynamics does take into account the velocity of the particles and therefore oscillates. Final Value of A-B = 0.7454 Å, B-C = 5.281 Å.
Question 4 - Reactive and unreactive trajectories
| p1 | p2 | Unreactive or Reactive |
|---|---|---|
| -1.25 | -2.5 | Reactive |
| -1.5 | -2.0 | Unreactive |
| -1.5 | -2.5 | Reactive |
| -2.5 | -5.0 | Unreactive |
| -2.5 | -5.2 | Reactive |
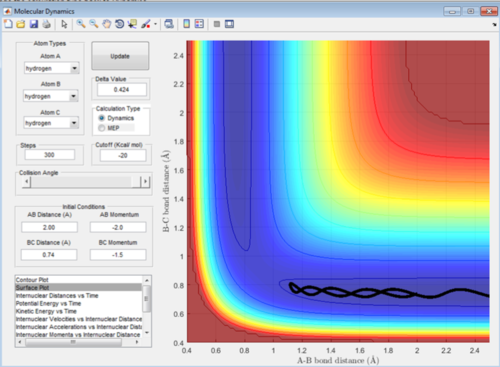
In this reaction trajectory the molecules overcome the activation barrier to react. The Ha approaches the Hb-Hc molecule decreasing its bond length and then upon reaction the Hb-Hc bond length increases as Hc moves away.
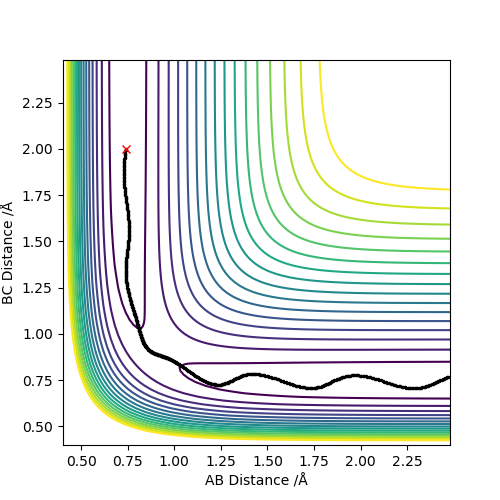
In this reaction trajectory the molecules do not overcome the activation barrier and therefore do not react.The Ha approaches the Hb-Hc but without enough energy to over come activation energy.
In this reaction trajectory the molecules overcome the activation barrier to react.
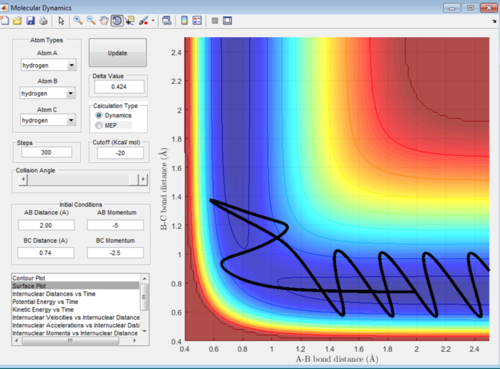
In this reaction trajectory the molecules overcome the activation barrier to react initially then return to be below activation energy and therefore do not end up reacting. This is due to the significant change in momentum.
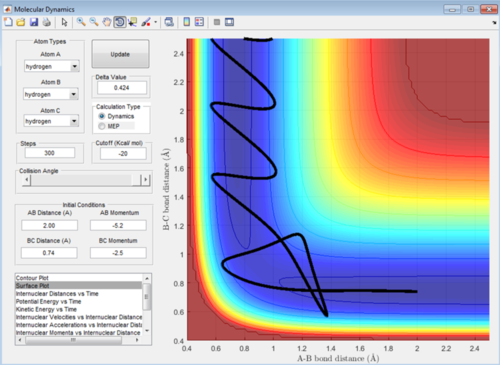
In this reaction trajectory the molecules overcome the activation barrier to react initially then return to be un-reacted and then overcome the activation barrier once more to react. This is due to the momentum
Question 5 - Transition State Theory
The transition state theory assumes equilibrium between the reactants and an activated transition state. Transition State Theory and experimental values will differ when transition states are very unstable and short lived as the momentum of the reaction trajectory of the reactants will affect that of the transition state and therefore the selectivity of the products.[1] Also this theory assumes that atomic nuclei behave according to classical systems and doesn't take into account the fact that nuclei can tunnel across the barrier without having the activation energy. As the transition state is a saddle point (a minimax point: in a 3D graph of internuclear distance of the reactants and products vs potential energy, it is a minima point between the internuclear distances but a maxima between internuclear distance and potential energy)[2] the reaction can also take alternative routes without having activation energy.
Exercise 2
F + H2 and FH + H
- Classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
- Locate the approximate position of the transition state.
- Report the activation energy for both reactions.
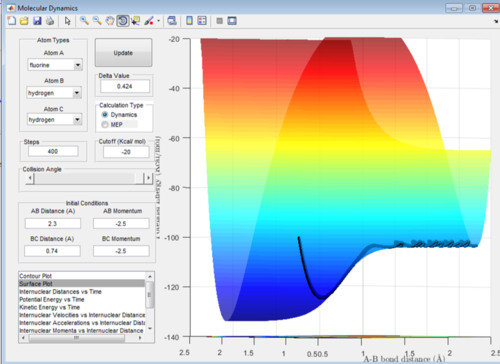
This reaction appears to be exothermic as the energy of the products is lower than that of the reactants. This bares true because it will be in a lower energy state upon forming a very strong H-F bonds.
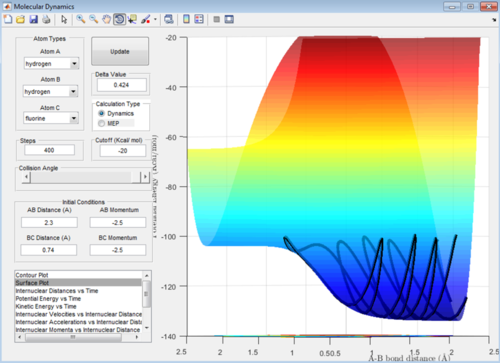
This reaction appears to be an endothermic reaction at the energy level of the products is a lot higher than that of the reactants. This bares true to the fact that he H-F bond is very strong and therefore will have a lower energy system.
When the transition state isn't obvious Hammonds postulate can be employed. It states that when the reaction is endothermic, the transition state represents that of the products as they are closer in energy to the transition state; if the reaction is exothermic the transition state represents that of the reactants as it is closer in energy to the maximum point on the energy diagram.[3]
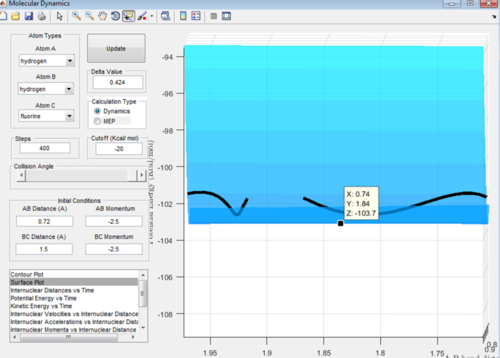
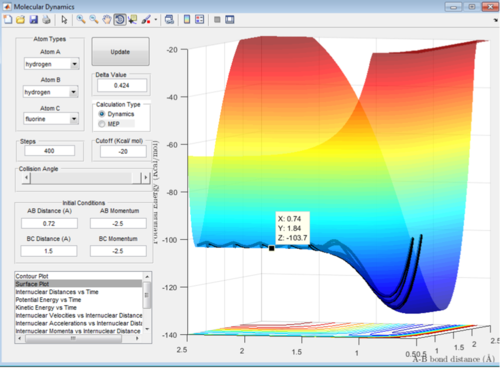
In this endothermic reaction it can be seen that the transition state is when the bond length between H-F is 1.84 Å and the bond length between H-H is 0.74 Å. It can be seen that this is very similar to that of the products as the slight parabola is very small and can be seen in Figure 11 by the change in gradient either side of the point on the line selected. The activation energy is: Energy of the transition state - Energy of reactants = -103.7-(-133.9 kcal/mol) = +30.2 Kcal/mol.
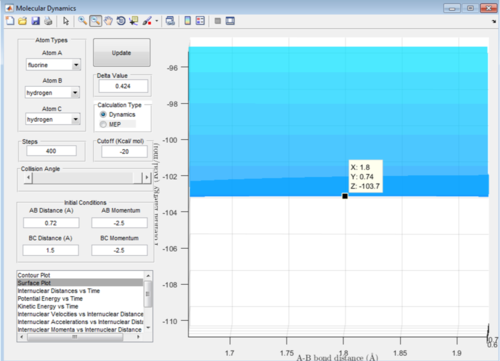
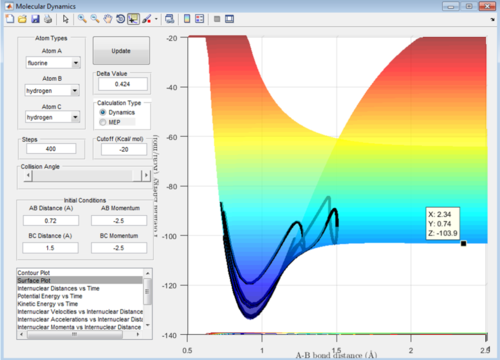
The values for this reaction should be very similar in terms of the transition state value. The activation energy is very small for this reaction: Energy of the transition state - Energy of reactants = -103.7-(-103.9)= + 0.2 kcal/mol Therefore this has a lot smaller activation energy than that of the other reaction due to the fact it is an exothermic reaction.
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. How could this be confirmed experimentally?
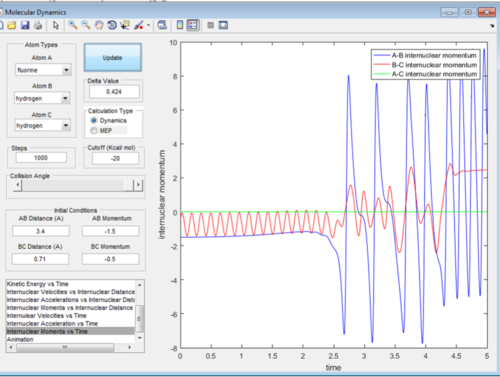
As the potential energy of the products is lower than that in the reactants in an exothermic reaction, the kinetic energy of the reactants must be lower than that in the products due to the fact that overall energy must be conserved. Due to the H-F bond being a lot stronger than the H-H bond it will have a stronger vibrational energy and therefore higher kinetic energy. This vibrational mode will be seen in an IR spectra. There will be one specific to the H-H bond and the H-F bond with the H-F bond being at a higher energy (higher wavenumber). From Figure 15 it can be seen that the magnitude of momentum is much lager in the A-B bond (H-F) than that of the B-C (H-H) bond meaning that also the kinetic energy is a lot larger.
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
Polanyi's empirical rules state that increased vibrational energy favours a late transition state, which occurs in an endothermic reaction where the products resemble the transition state. On the other hand, increased translational energy favours an early transition state, which occurs in an exothermic reaction where the reactants resemble the transition state.[4]
In the H-H + F reaction, as it is an exothermic reaction, high translational energy and low vibrational energy will provide a less efficient trajectory and therefore make the reaction less likely to go to completion. A increased momentum value is proportional to high translational energy and increased bond length is proportional to decreased vibrational energy. This relationship can be seen in Figure in 16 and 17, when the momentum was increased the trajectory was a lot less efficient and the reaction didn't go to completion.
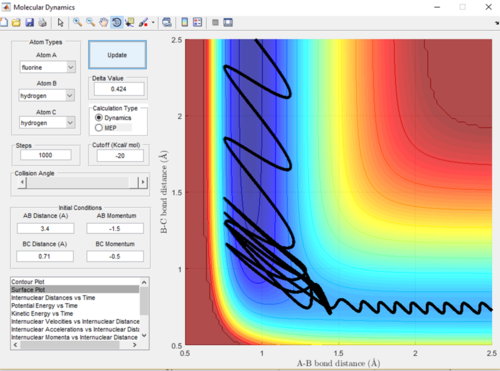
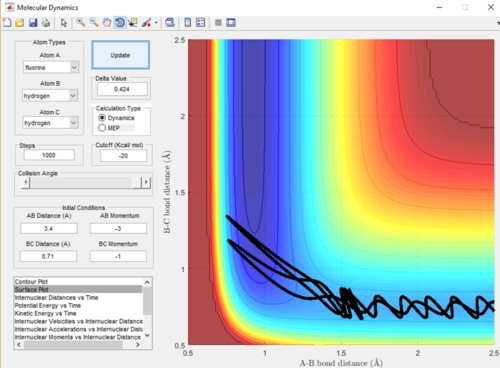
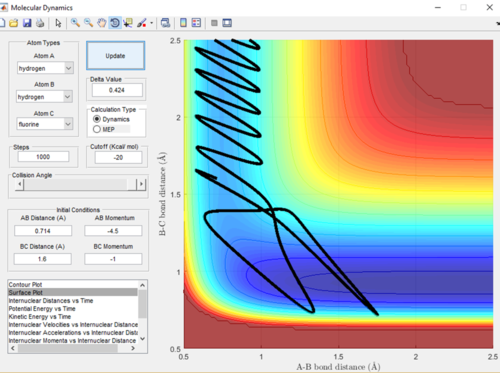
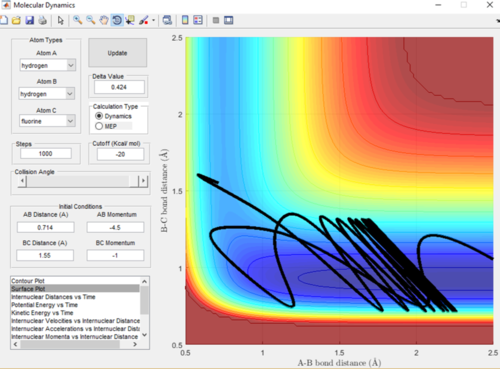
As the F-H + H reaction is endothermic it is therefore expected that a system with low translational energy but high vibrational energy will favour the transition state and give a less efficient trajectory and means the reaction is less likely to go to completion. A low momentum value is proportional to low translational energy and decreased bond length is proportional to increased vibrational energy. This relationship can be seen in Figure in 18 and 19, when the bond length was increased and therefore the vibrational energy decrease the trajectory was a lot more efficient and the reaction did go to completion.
Bibliography
- ↑ https://en.wikipedia.org/wiki/Transition_state_theory access date: 18.05.17
- ↑ https://en.wikipedia.org/wiki/Saddle_point access date: 18.05.17
- ↑ https://chem.libretexts.org/Reference/Organic_Chemistry_Glossary/Hammond%E2%80%99s_Postulate access date: 18.05.17
- ↑ "Theoretical Study of the Validity of the Polanyi Rules for the Late-Barrier Cl + CHD3 Reaction"; Zhaojun Zhang, Yong Zhou,† and Dong H. Zhang*; dx.doi.org/10.1021/jz301649w; J. Phys. Chem. Lett. 2012, 3, 3416−3419 © 2012 American Chemical Society; Available at URL: http://pubs.acs.org/doi/pdf/10.1021/jz301649w access date: 18.05.17
