Compwiki
Project molecule
NH3-ammonia
NH3 optimisation
N-H bond distance= 1.02 Å
H-N-H bond angle= 106°
The literature value of the bond N-H bond distance of NH3 is 1.008 ± 0.004 Å. [1] This is about the same value as the value found with Gaussian, the slight variation might be because accuracy to of 0.01 Å of the program.
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy (a.u.) | -56.55776873 |
| RMS gradient (a.u.) | 0.00000485 |
| Point group | C3V |
Item tabel
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 |
The final file of the optimisation is available here
Vibrations and charges
Vibrational frequency analysis
| Wavenumber cm-1 | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity arbitrary units | 145 | 14 | 14 | 1 | 0 | 0 |
| Image | 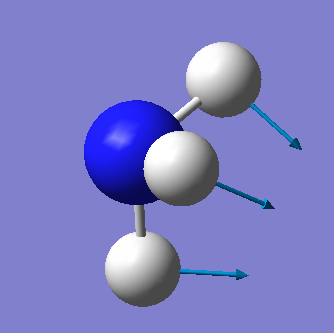 |
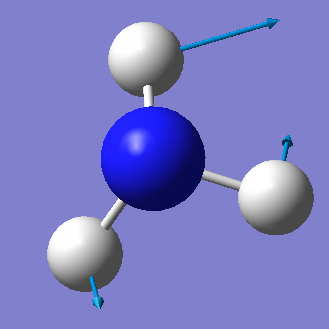 |
 |
 |
 |
|
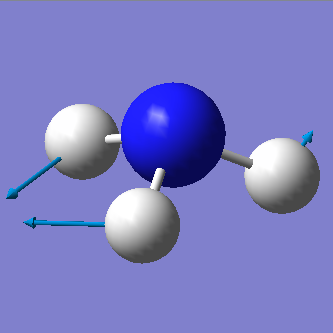
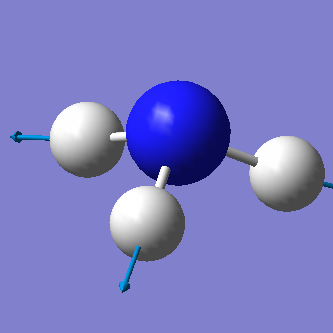
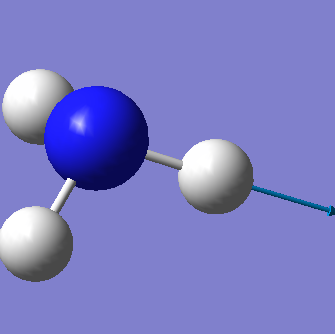
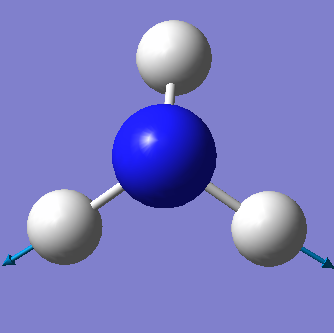
From the 3N-6 rule we would expect 6 vibrational modes (3x4-6=6). There are 3 bending vibrations (1090 cm-1, 1694 cm-1, 1694 cm-1) and 3 stretching vibrations (3461 cm-1, 3590 cm-1, 3590 cm-1). However, there are 2 degenerate bending vibrations (1694 cm-1) and 2 degenerate stretching vibrations (3590 cm-1).
The stretching vibration at 3461 cm-1 is highly symmetric because the point group of the molecule does not change during the vibration. The bending vibration at 1090 cm-1 is known as the "umbrella" mode.
In an experimental spectrum of gaseous ammonia we would expect to see 3 bands corresponding to the bending vibrations at 1090 cm-1 (strong band) and 1694 cm-1 and the stretching vibrations at 3461 cm-1 (weak band). Degenerate vibrations will not be observable since they are equal in energy and will therefore not be seen on the spectrum. The strecthing vibrations at 3590 cm-1 have a too weak intensity to be seen on the spectrum.
Atomic charges
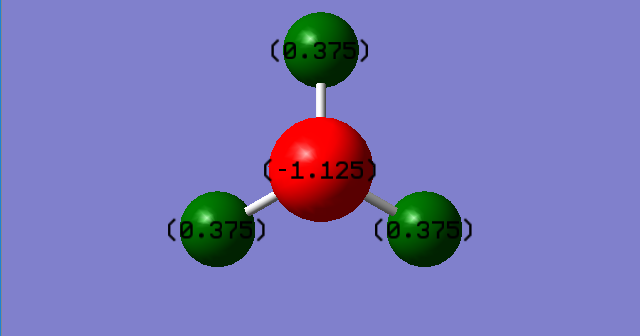
The charge of the N-atom is -1.125 and the charge of each H-atom is 0.375. The negative charge on the N-atom is as expected because of the lone pair of electrons on the N-atom creating a partial negative charge. Since a NH3 molecule is neutral, the partial negative charge on the N-atom must be balanced out by positive charges of the H-atoms.
N2-nitrogen
N2 optimisation
N-N bond distance= 1.11 Å
The literature value of the N-N bond distance is 1.0975 ± 0.0001 Å. [1] This is the same value found using Gaussian.
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy (a.u.) | -109.52412868 |
| RMS gradient (a.u.) | 0.00000060 |
| Point group | D*H |
Item table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N2 |
The final file of the optimisation is available here
Vibrations and charges
Vibrational frequency analysis
| Wavenumber cm-1 | 2457 |
| Symmetry | SGG |
| Intensity arbitrary units | 0 |
| Image | 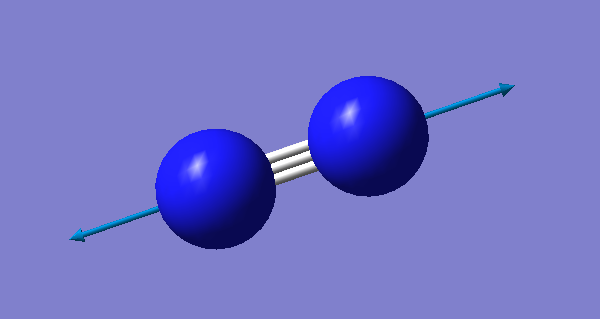
|
Since nitrogen is a linear molecule, it has 1 vibrational mode (3N-5=3x2-5=1). This is a stretching vibration at 2457 cm-1. However, this vibration is not visible on a spectrum because there is no change in dipole moment occuring during this vibration.
Atomic charges
Charges of N2
The nitrogen molecule is neutral and since both atoms are the same, we expect no charge on both atoms.
H2-hydrogen
H2 optimisation
H-H bond distance= 0.74 Å
This value is exactly the same as the literature value (74 pm). [2]
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy (a.u.) | -1.17853930 |
| RMS gradient (a.u.) | 0.00012170 |
| Point group | D*H |
Item table
Item Value Threshold Converged? Maximum Force 0.000211 0.000450 YES RMS Force 0.000211 0.000300 YES Maximum Displacement 0.000278 0.001800 YES RMS Displacement 0.000393 0.001200 YES
H2 |
The final file of the optimisation is available here
Vibrations and charges
Vibrational frequency analysis
| Wavenumber cm-1 | 4461 |
| Symmetry | SGG |
| Intensity arbitrary units | 0 |
| Image | 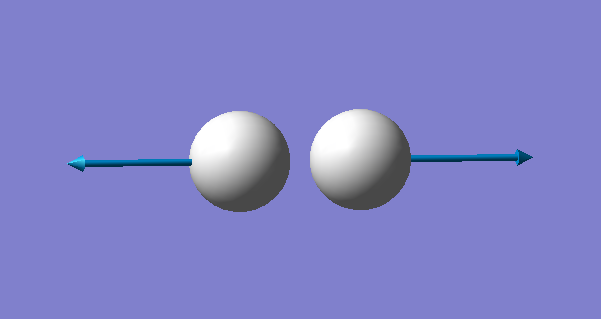
|
Since hydrogen is a linear molecule, it has 1 vibrational mode (3N-5=3x2-5=1). This is a stretching vibration at 4461 cm-1. However, this vibration is not visible on a spectrum because there is no change in dipole moment occuring during this vibration.
Atomic charges
Charges of H2
The hydrogen molecule is neutral and since both atoms are the same, we expect no charge on both atoms.
Structure and reactivity
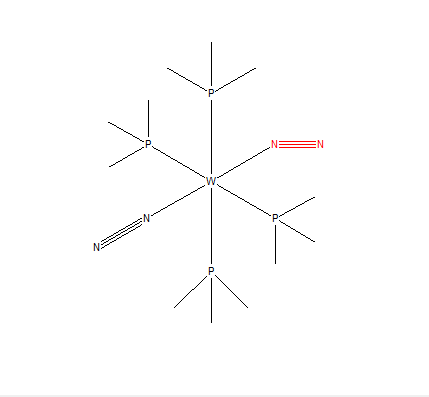
Mono-metallic complex coordinating N2
BUYBUS |
The mono-metallic complex that coordinates N2 was found to be trans-bis(dinitrogen)-tetrakis(trimethylphosphine)-tungsten. It can be identified by BUYBUS and its deposition number is 1117756. The bond lengths of the N-N bonds are 1.191 Å. This is a longer bond than the one found for N2 (1.106 Å). the reason for this longer N-N bond in the transition metal is the strength of the bond. Since the nitrogen atom has a positive charge because it has 4 bonds (as opposed to 3), there is less electron density surrounding the atom and therefore shared by the triple bond between the 2 nitrogens. This means that the bond is weaker and is thereby also longer.
Haber-Bosch process
The energy for the following reaction (the Haber-Bosch process) can be determined using the energies previously determined: N2 + 3H2 -> 2NH3
E(NH3)= -56.5577687 a.u.
2*E(NH3)= 2 * -56.5577687 = -113.1155374 a.u.
E(N2)= -109.5241287 a.u.
E(H2)= -1.1785393 a.u.
3*E(H2)= 3 * -1.1785393 = -3.5356179 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= 2625.5 [ (-113.1155374)-[-109.5241287-3.5356179] ] = 2625.5 * (-0.0557908) = -146.4787454 kJ/mol
The energy difference is -146.5 kJ/mol. This means that the ammonia product is more stable than the gaseous reactants since the energy difference is negative and the reaction is therefore exothermic.
Molecule of my choice: CO2-carbon dioxide
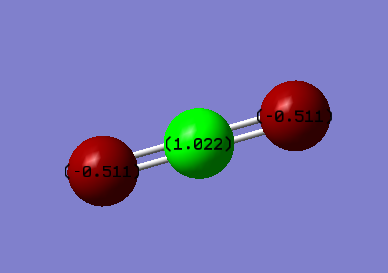
C=O bond distance= 1.26 Å
O=C=O bond angle= 180°
The literature value of the C=O bond distance in carbon dioxide is 1.163 Å. [3] This is a slightly smaller value from the one found using Gaussian. However, the error is so small that we can consider the value found to be correct.
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy (a.u.) | -188.58093945 |
| RMS gradient (a.u.) | 0.00001154 |
| Point group | D*H |
Item table
Item Value Threshold Converged? Maximum Force 0.000024 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.000021 0.001800 YES RMS Displacement 0.000015 0.001200 YES
CO2 |
The final file of the optimisation is available here
Vibrations and charges
Vibrational frequency analysis
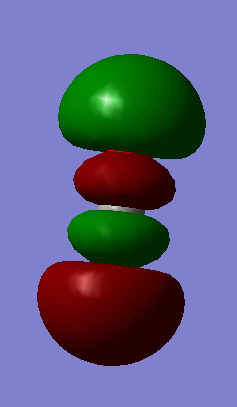
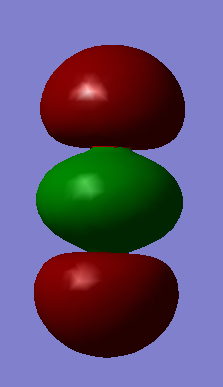
| Wavenumber cm-1 | 640 | 640 | 1372 | 2436 |
| Symmetry | PIU | PIU | SGG | SGU |
| Intensity arbitrary units | 31 | 31 | 0 | 546 |
| Image | 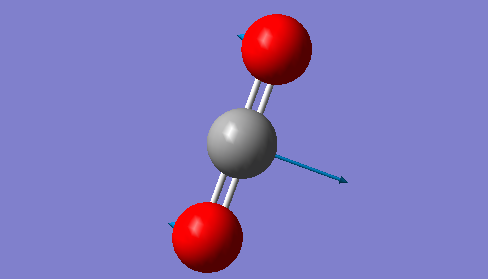 |
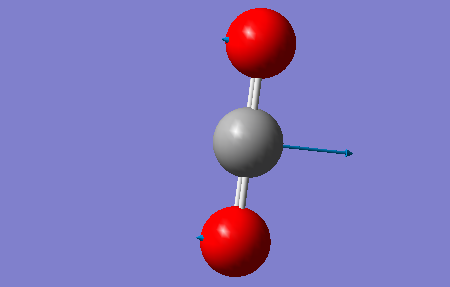 |
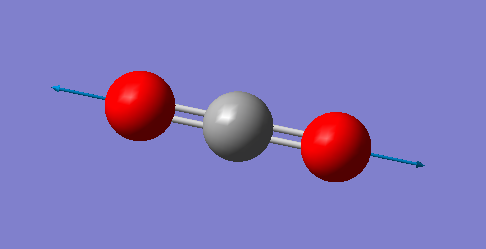 |
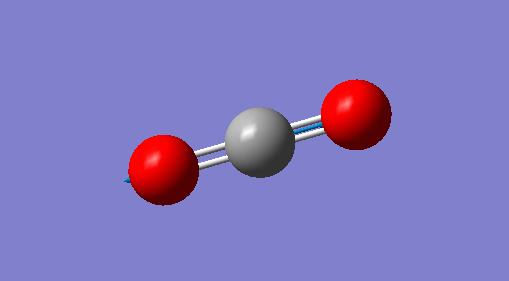
|
CO2 is a linear molecule which means that the 3N-5 rule is applied. Therefore, we expect to see 4 vibrational modes (3*3-5=4). The 2 vibrations at 640 cm-1 are degenerate bending vibrations. The vibration at 1372 cm-1 is a symmetric stretching vibration and the vibration observed at 2436 cm-1 is an asymmetric stretcing vibration.
On a spectrum, we expect to see 2 bands corresponding to the bending vibrations at 640 cm-1 and the asymmetric stretching vibration at 2436 cm-1. The two bending vibrations are degenerate which means that only one band will be observable for both vibrations. Furthermore, the symmetric stretching vibrations at 1372 cm-1 don't involve a change in dipole moment of the molecule which is why there is no band for this vibration on a spectrum.
Atomic charges
Charges of CO2
We expect a positive charge on the carbon atom and a negative charge on the oxygen atoms. Indeed, the carbon atom has a charge of 1.022 and the oxygen atoms each ahve a charge of -0.511. The oxygen atoms are more electronegative than the carbon atom which creates a dipole moment. Overall, the molecule is neutral, the negative charges on the oxygen atoms are compensated by the positive charge on the carbon atom (which is less electronegative than O).
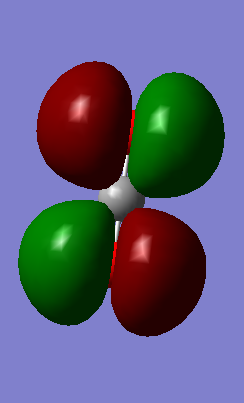
Molecular orbitals
References
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 0.5/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - however, only 2 bands are expected because the intensity of mode 4 is too low to be seen as well. The reason for the expected charges on the atoms are the different electronegativities rather than the localised electron pair.
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES - you included the unique identifier and the deposition number instead of the link directing to the crystal structure.
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES you presented the information in a good way - good job! The 4th displayed is a bonding one rather than non-bonding. To get the last mark you should have analysed the information in more detail and discuss the ordering of the MOs in terms of their energies.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or
YES - for future work try to find reference papers or books rather than webpages. However, the given webpage seems to be reasonable as it stated a common lecture book as reference. Additionally, you could have discussed the differences in more detail.
Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far