CompLab:ZCJ17
NH3
Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) E(RB3LYP) -56.55776873 a.u. RMS Gradient Norm 0.00000485 a.u. Point Group C3V
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986277D-10
Optimization completed.
-- Stationary point found.
NH3 |
The optimisation file is linked to here
Vibrations
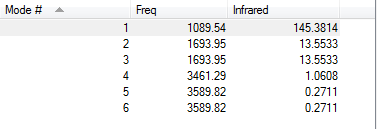
From the 3N-6 rule, you would expect there to be (3*4)-6 = 12-6 =6 vibrations. This is consistent with the vibrations outputted by Gaussian.
Modes 2 and 3 are degenerate (1693.95) as well as modes 5 and 6 (3589.82).
The first three modes are bend vibrations. The final three are stretch vibrations. Mode 4 is highly symmetric Mode 1 is known as the umbrella mode In an experimental spectrum there would be four bands, as two pairs of modes are degenerate.
| Atom | Charge (a.u.) |
|---|---|
| N | -1.125 |
| H | 0.375 |
This is approximately what is expected as the Nitrogen has an electronegativity of 3.04.[1], whereas the electronagtivity of hydrogen is 2.2.[1]. This means the Nitrogen is holding the electrons closer, therefore has more negative charge. The bond angle is 107 degrees.
N2
Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) E(RB3LYP) -109.52412868 a.u. RMS Gradient Norm 0.00000060 a.u. Point Group D*H Bond Length 1.10550 Angstrom
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.401075D-13 Optimization completed. Stationary point found.
N2 |
The optimisation file is liked to here
Vibrations
There are no negative vibrations
H2
Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) E(RB3LYP) -1.17853936 a.u. RMS Gradient Norm 0.00000017 a.u. Point Group D*H Bond Length 0.74279 Angstrom
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.164080D-13 Optimization completed. Stationary point found.
H2 |
The optimisation file is linked to here
Vibrations
There are no negative vibrations
Reaction Energy Calculations
E(NH3) = -56.55776873 a.u.
2*E(NH3) = -113.11553746 a.u.
E(N2) = -109.52412868 a.u.
E(H2) = -1.17853936 a.u.
3*E(H2) = -3.53561808 a.u.
ΔE= 2*E(NH3) - [E(N2) + 3*E(H2)]
= -113.11553746 - [ -109.52412868 + (-3.53561808 )]
= -113.11553746 - [ -113.05974676]
= -0.05579070 a.u.
= -146.49 kJ/mol
The reaction is exothermic and gives out energy, so therefore the products are more stable as they are of lower energy.
S2
Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) E(RB3LYP) -796.32599779 a.u. RMS Gradient Norm 0.00000372 a.u. Point Group D*H Bond Length 1.92943 Angstrom
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000011 0.001800 YES RMS Displacement 0.000016 0.001200 YES Predicted change in Energy=-7.077703D-11 Optimization completed. Stationary point found.
S2 |
As it is a diatomic molecule there is no charge on either of the atoms.
The optimisation file is linked here
Vibrations
There are no negative vibrations
Molecular Orbitals
The electronic configuration of a Sulfur atom is 1s2 2s2 2p6 3s2 3p4
This is the first molecular orbital. It contains two electrons, but they are pulled very deep in energy (-88.93663 a.u./-233503.13985 kJ/mol) so they are not involved in bonding.
This is the 11th molecular orbital. It was formed by combiing 2 3s orbitals and contains two electrons
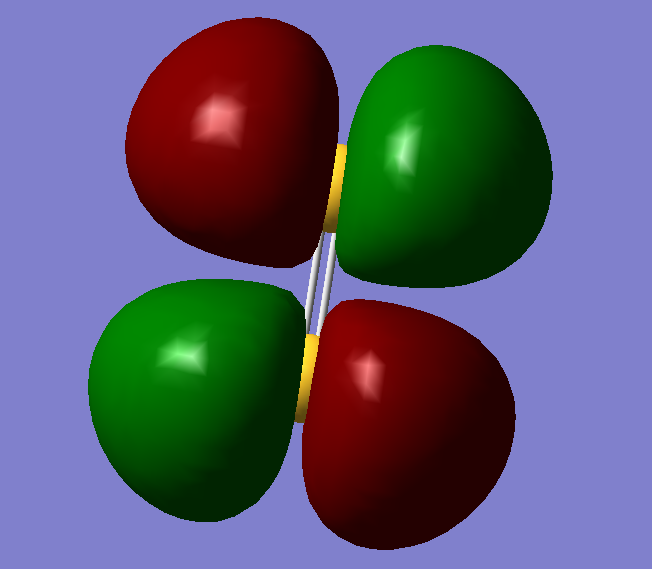
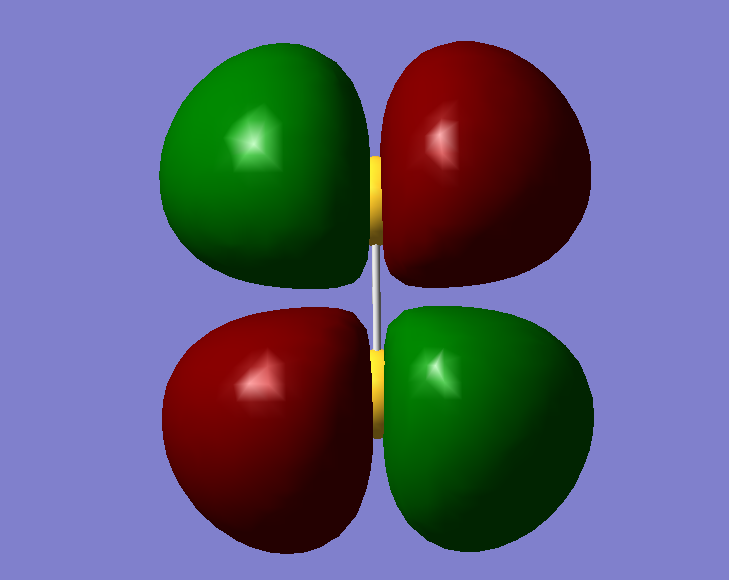
This is the fifteenth molecular orbital. It contains two electrons and is a pi orbital. It is involved in bonding as there is a very good overlap.
This is the HOMO- the Highest Occupied Molecular Orbital. It is an antibonding orbital and contains two electrons
This is the LUMO- the Lowest Unoccupied Molecular Orbital. It is not involved in bonding and contains no electrons
Comparison of Gaussian vs Literature Values
Literature Values taken From Atkins
| Atom | Property | Gausssian | Literature.[1] |
|---|---|---|---|
| N 2 | Bond Length | 1.10550 Angstrom | 1.0976 Angstrom |
| H 2 | Bond Length | 0.74279 Angstrom | 0.74138 Angstrom |
This shows that Gaussian produces results very close to the accepted values.