Cfl:rg8791
Module 1
The Basic Techniques of Molecular Mechanics and Semiempirical Orbital Molecular Methods for Structural and Spectroscopic Evaluations
Modelling Using Molecular Dynamics
Section 1: The Hydrogenation of Cyclopentadiene Dimer
The energies of both dimers and dihydro derivatives were minimised using the MM2 force field option; results tabulated below.
Comparing Structures 1 and 2
Cyclopentadiene dimerises to give two products as shown in the reaction scheme below.
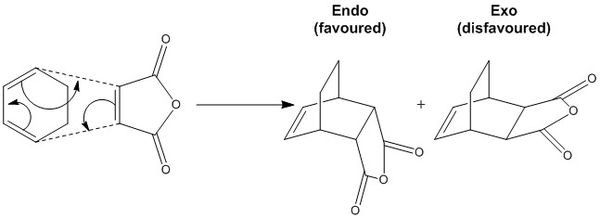
Despite the higher energy of molecule 2, the formation of the endo-dimer is observed which suggests that the dimerisation is kinetically rather than thermodynamically controlled. The exo-product has a lower energy due to less steric hindrance, however the endo-product has a more favourable transition state and therefore a lower activation energy. The endo-transition state is stabilised by a greater p orbital overlap due to a staggered ,rather than aligned, approach of the reacting molecules[1].
| Energies (kcal/mol) | Molecule 1 | Molecule 2 |
|---|---|---|
| Stretch | 1.2802 | 1.2507 |
| Bend | 20.6011 | 20.8656 |
| Stretch-Bend | -0.8359 | -0.8304 |
| Torsion | 7.6544 | 9.5035 |
| Non-1,4 VDW | -1.4323 | -1.5162 |
| 1,4 VDW | 4.2365 | 4.2936 |
| Dipole/Dipole | 0.3774 | 0.4452 |
| Total Energy | 31.8814 | 34.0120 |
Comparing Structures 3 and 4
The relative contributions from the stretching, bending, and van der Waals are greater for compound 3 than compound 4 which shows molecule 3 to be more stable. The greatest difference in energy contributions is seen with the bending energy, the large increase is due to increased steric hindrance as the double bond is located in a smaller ring system. Based on this data, the thermodynamic prediction appoints molecule 4 the major product.
| Energies (kcal/mol) | Molecule 3 | Molecule 4 |
|---|---|---|
| Stretch | 1.2659 | 1.1028 |
| Bend | 19.8063 | 14.5591 |
| Stretch-Bend | -0.8276 | -0.5493 |
| Torsion | 10.8698 | 12.4947 |
| Non-1,4 VDW | -1.2207 | -1.0908 |
| 1,4 VDW | 5.6394 | 4.4970 |
| Dipole/Dipole | 0.1621 | 0.1470 |
| Total Energy | 35.6953 | 31.1541 |
Section 2: Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
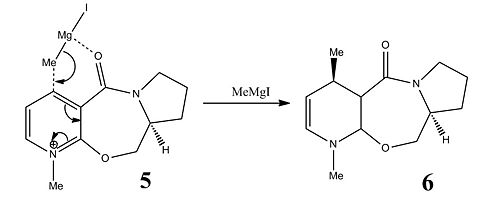
The first reaction, shown above, involves the optically active derivative of prolinol (5) which reacts with methyl magnesium iodide to alkylate the pyridine ring in the 4-position via a 6-membered transition state[2]. Since the Mg coordinates to the oxygen in the carbonyl group, the grignard reagent attacks from the same side as the carbonyl and this yields a product with absolute stereochemistry shown in 6.
The MeMgI component could not be included in the calculations because ChemBio3D does not recognise the element Mg, and calculations cannot be run on two molecules at once due to the limitations of the programme.
The orientation of molecule 5 was manually changed and the energy minimised using MMFF94 - it was possible to change the change the geometry of the carbonyl group relative to the aromatic ring but there was only a small variation between the energies. The lowest energy conformation had a dihedral angle of 8.2119 degrees. Three examples and associated energies are shown below.
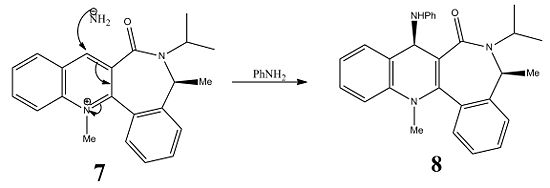
In the second reaction, the pyridinium derivative(7) reacts with aniline to form 8. Product 8 shows the -NHPh group to be above the aromatic ring; NH2PH attacks from the top face as the bottom face is more sterically hindered due to downward angle of the carbonyl group(derivative 7 is the lower energy conformer c.f. the carbonyl above the ring). Molecule 8 is atropisomeric; steric hindrance around a single bond causes the rotation around the bond to be sufficiently slow so that conformers are separable.
The molecular mechanics modelling technique used in this exercise takes into account basic lone pair repulsions, sterics and van der waals forces, an improved model could include molecular orbital interactions by using the MOPAC molecular orbital method.
Section 3: Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
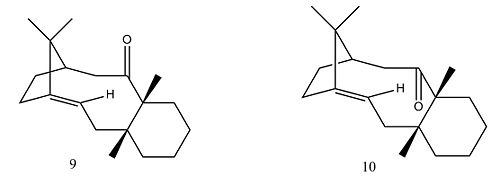
In the synthesis of Taxol, a key intermediate with the carbonyl group pointing up (9) or down (10) is initially synthesised. By modelling the possible structures in ChemBio3D and running MM2 and MMFF94 energy minimisations, intermediate 10, with the carbonyl group pointing downwards, was determined to be the most stable.
| Energies (kcal/mol) | Molecule 9 | Molecule 10 |
|---|---|---|
| Stretch | 2.7954 | 2.6279 |
| Bend | 13.4072 | 14.9075 |
| Stretch-Bend | 0.6464 | 0.2909 |
| Torsion | 22.2379 | 19.6974 |
| Non-1,4 VDW | -1.7221 | -1.0682 |
| 1,4 VDW | 15.5844 | 13.4264 |
| Dipole/Dipole | 0.000 | 0.0174 |
| Total Energy | 52.9493 | 49.8994 |
Molecule 10 is of lower energy throughout all the energy break downs except for the van der waals and dipole-dipole interactions. The higher energy of molecule 9 is due to the cyclohexane ring pointing upwards so that when the carbonyl is also pointing upwards this causes steric hindrance.
It is difficult to lower the energy any further by manual distortion since the calculations often fall into local minima. The lowest energy conformers are those with the cyclohexane ring in a chair conformation and adjusting hydrogens to resemble sp3 figuration. Left standing the compound will isomerise to the carbonyl conformer with the lowest energy, which is a further example in this project of atropisomerism.
The alkene is known to react unusually slowly as it is a hyperstable alkene. [3]Hyperstable alkenes have negative strain energies so that the strain energy is less than the corresponding parent hydrocarbon. This leads to lower heats of hydrogenation than normal; an increased driving force would be needed to overcome the hyperstability.
Modelling Using Semi-empirical Molecular Orbital Theory
Section 4: Regioselective Addition of Dichlorocarbene
The molecular orbitals of compound 12 - shown left - were generated to further understand the reactivity demonstrated by the compound. The molecule's structure was first optimised using the MM2 field method (classical molecular method) and then the MOPAC/PM6 method was run to approximate the wave function of valence electrons (quantum method). The initial MM2 calculation found molecule 12 to have an energy of 17.8993 kcal/mol.
| HOMO | HOMO-1 | LUMO | LUMO+1 | LUMO+2 |
|---|---|---|---|---|
 |
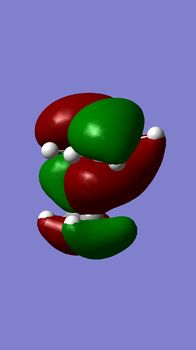 |
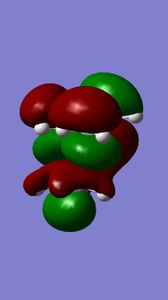 |
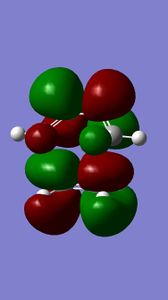 |
|
Discussing MOs
Compound 12 reacts endo to the chlorine substituent on the opposite face of the cyclopropyl ring[4], the reason for this can be seen from analysing the molecular orbital diagrams.There is an anti-periplanar overlap with Cl-C sigma* (LUMO+2) orbital which results in the exo pi-orbital (HOMO-1) being stabilised relative to the endo pi-orbital [5]. In addition, the HOMO-1 orbital is more diffuse. This antiperiplanar stabilising interaction therefore renders the endo bond more nucleophilic in terms of both the frontier orbital and electrostatic sense as the endo double bond is further from the electrophilic chlorine which pulls electron density towards itself. The HOMO orbital, shown above, supports this as greater electron density around the endo double bond can be seen compared to the exo double bond. In all, the endo double bond is rendered more reactive.
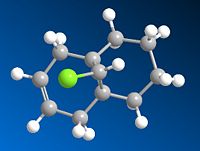
Compound 12 was reacted with dichlorocarbene to produce compound 13 (left), IR analysis of compound 12 and 13 were run and compared to literature values and each other. The results are tabulated below.
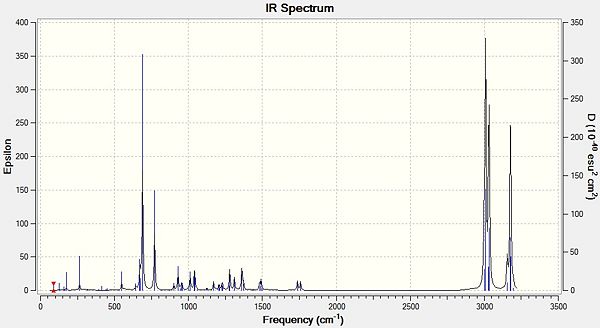
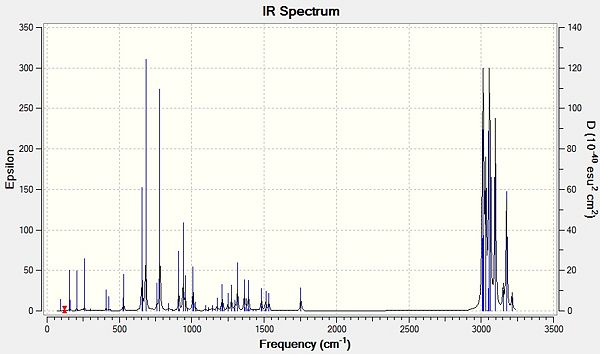
| Molecule | Anti Alkene stretch (cm-1) | Syn Alkene Stretch (cm-1) | C-Cl Stretch (cm-1) |
|---|---|---|---|
| Dialkene (12) | 1737.10 | 1757.37 | 770.83 |
| Monoalkene (13) | n/a | 1753.76 | 779.90 |
| Literature [5] | 1690-1630 | 1690-1630 | 785-540 |
Discussing IR
The effect of the chlorine group on the two alkenes in compound 12 can be quantified by analysing the molecule’s vibrations using the density functional approach. As expected, the two absorptions for the alkene groups in compound 12 differ; the alkene group syn to the chlorine absorbs at a higher wavelength indicating that this is a stronger bond than the anti alkene group, as more energy is required to make the atoms vibrate (E=hv). This observation corresponds with the reported reactivity, as the weaker alkene bond breaks as the dichlorocarbene adds on, suggesting that this bond is weaker. The C-Cl stretch in the monoalkene increases slightly indicating that the strength of the C-Cl bond also increases upon hydrogenation. The syn alkene also increases in bond strength but by a lesser amount, suggesting that the syn-alkene is less affected by the anti-alkene.
All stretches lie near literature values but not completely within the range, this can be explained by taking into account the limitations of the model used to calculate the vibrations. Firstly, the programme approximates the calculations so that the vibrations are considered as harmonic oscillators rather than anharmonic; a more accurate prediction could be made by including anharmonicity in the calculation. Secondly, the calculations are performed within a finite set of basis functions, therefore changing the basis set could yield more accurate results.
IR spectra of Compound 12 (top) and compound 13 (bottom) are shown below.
Mini-Project:Computational Investigation of the Diels-Alder Cycloadditions of 4-Chloro-2(H)-pyran-2-one
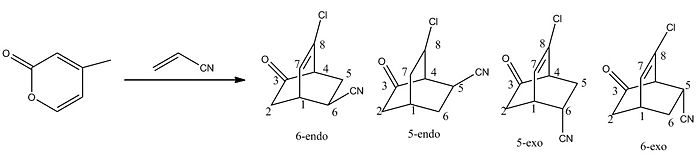
4-Chloro-2(H)-pyran-2-one undergoes Diels-Alder cycloaddition with electron-deficient dienophiles to give 6-endo- and 5-endo-bridged bicyclic lactones which usually cannot be isolated. This particular project was chosen as the reaction (left) there is a mixed ratio of regio- and stereo-isomers.
The 13C-nmr spectra will differ for the 5- and 6-substituted compounds and analysis of the thermodymanic energies of the molecules could distinguish between exo- and endo-isomers, endo being of lower energy. Note, this project examines the 13C-nmr but to further investigate this reaction the vibrations of each molecule could be examined. The 13C spectra for each isomer was generated using the GIAO method and compared to literature values[6] if available. The NMR data is tabulated below, see above diagram for labelled atoms.
| Atom | Literature Shift /ppm | Calculated Shift /pmm | Difference/ ppm |
|---|---|---|---|
| 3 | 169.0 | 166.1 | 2.9 |
| 8 | 138.0 | 140.4 | 2.4 |
| 7 | 124.9 | 125.9 | 1 |
| CN | 118.3 | 113.7 | 4.6 |
| 1 | 73.4 | 77 | 3.6 |
| 4 | 48.3 | 52.1 | 3.8 |
| 6 | 29 | 33.5 | 4.5 |
| 5 | 26.5 | 30.9 | 4.4 |
| Atom | Literature Shift /ppm | Calculated Shift /pmm | Difference/ ppm |
|---|---|---|---|
| 3 | 167.5 | 165.2 | 2.3 |
| 8 | 133.4 | 135.4 | 2 |
| 7 | 128 | 130.1 | 2.1 |
| CN | 117.9 | 113.8 | 4.1 |
| 1 | 73.3 | 76.8 | 3.5 |
| 4 | 50.6 | 54.6 | 4 |
| 6 | 31.9 | 36.1 | 4.2 |
| 5 | 22.9 | 27.8 | 4.8 |
| Atom | Literature Shift /ppm | Calculated Shift /pmm | Difference/ ppm |
|---|---|---|---|
| 3 | 168.7 | 166.1 | 2.6 |
| 8 | 137.0 | 139.2 | 2.2 |
| 7 | 125.2 | 127.7 | 2.5 |
| CN | 118.1 | 114.3 | 3.8 |
| 1 | 73.8 | 78.4 | 4.6 |
| 4 | 47.9 | 52.1 | 4.2 |
| 6 | 28.9 | 33.1 | 4.2 |
| 5 | 25.0 | 29.3 | 4.3 |
| Atom | Calculated Shift /pmm |
|---|---|
| 3 | 164.3 |
| 8 | 136.6 |
| 7 | 130.8 |
| CN | 113.8 |
| 1 | 76.4 |
| 4 | 54.7 |
| 6 | 35.8 |
| 5 | 28.7 |
The carbon-13 NMR matches well to the literature references; all chemical shifts are within 5 ppm of the reported shifts. The small difference is observed due to the limitations of the computational method used; the structure drawn is treated as an absolute structure (with no flexibility) where as in reality, a lab sample may consist of a slightly different conformer e.g. the positions of 2 atoms in the ring may be different although the compound is still exo or endo.
The selectivity is reported as follows; 6-endo (58%), 5-endo (25%), 6-exo (17%), and 5-exo (0%)[6]. The cycloaddition resembles the mechanism illustrated in section two (the hydrogenation of the cyclopentadiene dimer), whereby the exo and endo isomers are determined by a staggered or aligned approach. Thus due to the stabilised transition state discussed above, the endo isomer is favoured. The 6-substitution is reported to be favoured over the 5-substituted product; this may be due to the distance of -CN to the chlorine group, MO analysis would be needed to provide a sound conclusion as to why this is. To further this project, molecular vibrations could also be examined.
References
- ↑ Clayden, Greeves, Warren, and Wothers, Organic Chemistry, 2008 912
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838
- ↑ W Maier and P Scleyer, J. Am. Chem. SOC. 1981, 103, 1891-1893
- ↑ B. Halton and S. G. G. Russell, J. Org. Chem., 1991,56, 5553
- ↑ 5.0 5.1 B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447 Cite error: Invalid
<ref>tag; name "five" defined multiple times with different content - ↑ 6.0 6.1 K Afarinkia, M Bearpark, and A Ndibwami,J. Org. Chem. 2003, 68, 7158-7166
