Cbl08:module 1
Module 1: Organic
The Hydrogenation of Cyclopentadiene Dimer
Diels-Alder Cycloaddition: Dimerisation of Cyclopentadiene
Cyclopentadiene can undergo self 4πs +2πs Diels-Alder cycloaddition to form product with two possible conformations: the endo and the exo product. From the molecular calculation ( MM2 force field in ChemBio 3D Ultra) as shown in table 1.1i, the endo product is thermodynamically less stable than that of the exo product and the differece is largely contributed by the torsion strain in the endo dimer. This increase in torsion strain is most likely due to the steric interaction between the 6-membered ring with carbon bridge and the 5-membered ring.
| Energy/kcal mol-1 | Exo Product (1) | Endo Product (2) | Hydrogenated Product (3) | Hydrogenated Product (4) |
|---|---|---|---|---|
| Bend | 20.5593 | 20.8436 | 18.9520 | 14.5538 |
| Torsion | 7.6657 | 9.5132 | 12.1125 | 12.5029 |
| 1,4 VDW | 4.2424 | 4.3237 | 5.7159 | 4.4976 |
| Total Energy | 31.8814 | 33.9987 | 35.9410 | 31.1658 |
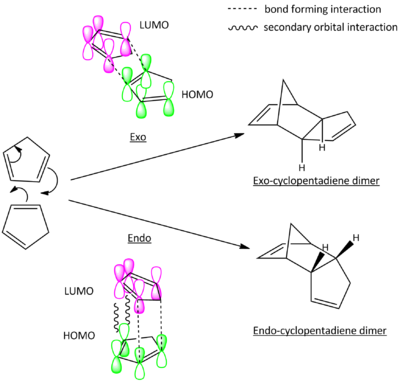
Though the exo product is thermodynamically favoured, it is shown experimentally that the reaction yields exclusively the endo product.[1] By considering the frontier molecular orbitals of the reactants, the endo product is kinetically favoured due to secondary orbital stabilisation as shown in Fig.1.1b. In the endo transition state shown in fig. 1.1b, the HOMO of the dienophile will interact more favourably with the LUMO of the diene due to the secondary orbital stabilisation arise from better orbital overlap. This lowers its activation pathway and therefore the endo product is kinetically favoured.
Hydrogenation of Endo Cyclopentadiene Dimer
The unsaturated cyclopentadiene dimer can undergo further hydrogenation through H2, Pd/C, with possible attacks on two different sites: the C=C bond in the 5-membered ring or the C=C bond in the norborene ring as shown in Fig. 1.1c.
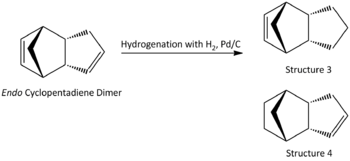
From the thermodynamics calculations in Tabla 1.1, structure 3 raises the total energy of the molecule relative to the unhydrogenated endo dimer, whereas structure 4 is lower in energy and therefore it is thermodynamically more favourable.
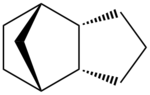
The main contribution to the difference in energy between structure 2 and structure 3/4 can be attributed to torsion and bending. Hydrogenation of C=C bond reliefs bending strain significantly but increases torsion strain as a result of carbon centre going from sp2 to sp3 with additional hydrogen atoms. In structure 3, hydrogenation of C=C bond in the 5 membered ring will force the added hydrogen atoms to be eclipsing each other due to ring conformational restriction and hence increase steric strains. Whereas for structure 4, the bending relief is much more significant in structure 4 than 3 because the ring strain is relieved with respect to the bridging carbon and the reduced C-C bond can rotate more freely. With reference to the literature [2], initial hydrogenation of C=C bond in noborene ring is favoured over that in the 5 membered ring. The double bond in the 5 membered ring will be reduced upon further reduction as shown in Fig. 1.1d.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
Nucleophilic Addition of Prolinol with Grignard Reagent, MeMgI
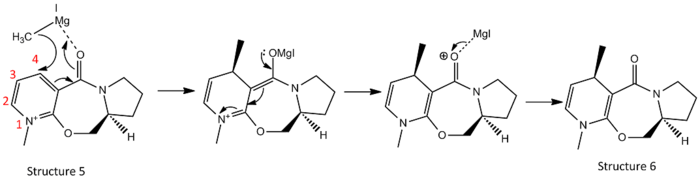
Methylation of pyridine rign can be highly stereo- and regioselective through nucleophilic addition with Grignard reagents. The methyl group is expected to add specifically at the C(4) position of the pyridine ring due to chelate effect from the magnesium and the carbonyl group. Selectivities as high as 99:1 is observed experimentally with bulkier halide substituent such as iodide in the Grignard reagent.[3] From the product shown in structure 6, there are two chiral centres: one at the alkylating centre and one at the hydrogen anti to the alkylated carbon.
In order to explain the stereoselectivity, the position of the carbonyl group relative to the alkylating carbon centre is crucial and hence the dihedral angle between the C=O bond and C-H bond, and the conformation minima of the reactant has first to be determined.
To find potential different dihedral angles between the carbonyl bond and the aromatic ring under minimised energy of Prolinol structure 5, the MM2 energy minimisation was first carried out on automatic geometry after inputted the structure. A first set of data was achieved with dihedral angle of 9.0°. The molecule was then manually distorted with both oxygen in the molecule pulled 90° below the π-system and the result had no significant differences between the optimised energy and the dihedral angle. Hence the same optimisation was done with MMFF94 force field calculation and the results were tabulated as follow:
| Calculation Type | Pre-Opt Structure | Dihedral Angle/o | Energy/kcal mol-1 |
|---|---|---|---|
| MM2 | Automatic input structure | 9.0 | 43.1301 |
| MM2 | Both Oxygen 90o below the pi-system | 11.5 | 43.1436 |
| MMFF94 | Automatic input structure | -1.0 | 57.4874 |
| MMFF94 | Both Oxygen 90o below the pi-system | 13.2 | 57.4256 |
| MMFF94 | Carbonyl O 90o below the pi ring and enol O 90o above the ring | 19.7 | 58.7154 |
| MMFF94 | System widely stretched and strained | 36.4 | 59.1591 |
- The magnesium atom is excluded from modelling as the molecular mechanics/energy minisation was unable to calculate with metal atoms.
From the calculations of MMFF94, two conformation minima were found with dihedral angle of -1.0° and 13.2°. This is in good agreement with the literature[3] in which one conformer has the carbonyl group and the pyridinium ring coplanar, and the other conformer has the carbonyl group above the plane of the pyridinium ring and anti to the hydrogen at the other chiral centre. Taking either of these two conformers as the starting molecule, the incoming magnesium in the Grignard reagent will chelate onto the carbonyl oxygen from above the plane. This chelate effect restricts the position of the methyl group, allowing it only to attack from the top of the plane which is anti to the chiral hydrogen.
Nucleophilic Addition to Pyridinium Ring with Aniline
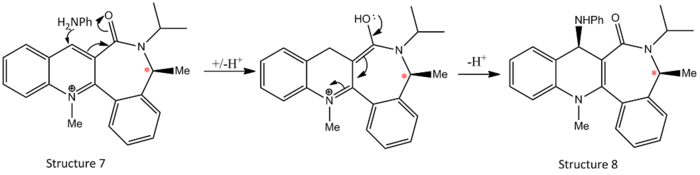
Methylation onto molecule 7 displays a total opposite stereochemistry with the aniline attacks anti to the carbonyl. In this reaction scheme, there is no Grignard reagent present to chelate onto the carbonyl group and hence steric factor dominates in this reaction.
Regardless of the distortion extent of the starting configurations, the molecule optimized into similar structures, with the carbonyl group syn to the chiral centre as marked in *.Without the presence of chelate effect, the incoming aniline favours to attack on the face anti to the carbonyl group for both steric and electronic considerations. The aniline has a bulky phenyl group attached and hence it attack from anti face minimised steric clashes. Also, the presence of phenyl group makes the aniline a highly electron rich nucleophile and hence anti attack is favoured to reduce electronic repulsion.
| Calculation Type | Dihedral Angle/o | Energy/kcal mol-1 |
|---|---|---|
| MMFF94 | -35.0 | 99.6127 |
| MMFF94 | -37.2 | 99.6799 |
The simulation ran by ChemBio 3D program is a thermodynamic approach that explains the relative energies of the molecules. However, this is insufficient to have a full understanding of a reaction as reaction depends on both kinetics and thermodynamics. To improve the model, the transition state of the molecule can be studied and the relative energy and orbital alignment of respective HOMOs and LUMOs can also be taken into consideration to determine the preferred outcome.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Introduction
Atropisomers are stereoisomers that have high kinetic barrier for rotating its single bond during isomer interconversion. This type of isomerism is commonly found in molecules with large rings in which its steric strains impose by the ring also play a part in rising its kinetic barrier for interconversion.
Results and Analysis
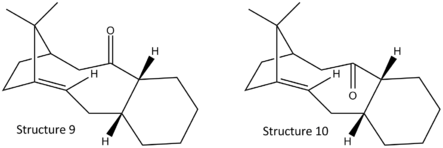
In this example, two atropisomers are found in the intermediate of taxol synthesis with restricted bond rotation in C-C=O bond as shown in Fig. 1.3a. To determine the most thermodynamically stable isomers, both structures were optimised with MM2 and MMFF94 force field.
Upon minimising structure 9 and 10, the optimised structures are both isomers with cyclohexane ring in twistboat conformations. This example is hence further investigated with the cyclohexane ring manually manipulated into chair conformation and the relative energy data were tablulated as shown in Table 1.3i:
| Structure | MM2 Energy/kcal mol-1 | MMFF94 Energy/kcal mol-1 |
|---|---|---|
| 9-Twistboat | 54.3865 | 79.3064 |
| 9-Chair | 48.8929 | 70.5486 |
| 10-Twistboat | 58.7616 | 81.5824 |
| 10-Chair | 53.1676 | 74.8324 |
From the 2D structure, structure 10 seems to be thermodynamically more stable than 9 due to lesser steric clashes between the bridging carbon and the carbonyl oxygen. However when the structure is modelled in 3D, the other end of the norborane ring tends to fold down and inwards, making the two methyl groups on the bridge pointing outwards and hence relieves the steric strains. This observation is supported by the modelling results where structure 9 in chair conformation is the most thermodynamically stable isomer whereas isomer 10 with twistboat conformation is the least stable isomer with highest energy. Isomers in structure 9 experience instability that arises from the steric clash between the oxygen and the bridging carbons. Similar steric clashes are also present in structure 10 isomers where the downward facing carbonyl oxygen clashes with the carbons in norborane ring. On top of that, structure 10 isomers also experience steric clash with the β–carbon in the cyclohexane ring which is closer and hence stronger steric interactions. This interaction is more significant in twistboat conformation than the chair conformation. Therefore if given enough energy to rotate the bond, structure 10 will isomerise to the more stable structure 9 as it is thermodynamically favourable.
For further addition to the carbonyl bond, the incoming group will attrack from Burgi- Dunitz angle of 109° to the carbon. From the 3D view of the molecules, addition will occur more favourably for structure 9 than 10 as the Burgi-Dunitz angle for 9 is spatially more available whereas that for 10 is more hindered by the hydrogens on the cyclohexane ring and bridging carbons.
Further Discussion: Reaction at Bridgehead C=C double Bond
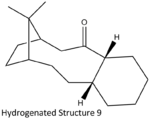
Both atropisomers studied includes a bridgehead double bond and a medium size polycyclic ring system. This double bond display unusually high stability and is regarded as a hyperstable alkene.[4] A hyperstable alkene is thermodynamically more stable than its hydrogenated derivative and hence reluctant to undergo hydrogenation. The reluctance of hydrogenation can be attributed to as suggested in two literatures [4] [5] that the cage structure adds extra stability to the alkene bond and the carbon centre will carbon centre will experience more strains when it goes from sp2 to sp3 in this conformationally restricted ring system.
A simple way to justify is to compare the optimised energy of the hydrogenated specie and its parent compound. The twistboat conformation of structure 9 is hydrogenated and it has a higher energy of 65.9243kcal mol-1.
Modelling Using Semi-empirical Molecular Orbital Theory
Part 1: Regioselective Addition of Dichlorocarbene
Predicting the major product in a reaction with classic mechanic parameters such as thermodynamics and sterics can sometimes proof to be limited, for example in a kinetically controlled reaction. As such, understanding energies of the molecular orbital that is built on quantum mechanics will be useful to learn about the electron donating /accepting power of the specie and the kinetically favoured pathway.

This example investigates the electrophilic addition of dichlorocarbene with structure 12 as shown above. In structure 12, two nucleophilic sites of C=C bond are susceptible to electrophilic addition from potential electrophile such as dichlorocarbene. This electrophilic addition reaction involves a flow of electron density from the nucleophile to the electrophile during bond formation and hence it is interesting to investigate the HOMO-LUMO interaction in this process.
Using the MOPAC Interface followed by PM6 calculation method, the wavefunction of structure 12 is calculated and the relevant molecular orbitals were modelled as shown in the figures below.
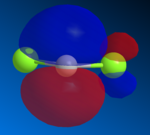
With the HOMO of the nucleophile has its largest electron density localised in the endo alkene bond and the LUMO of the dichlorocarbene eletrophile in the largely p-based antibonding orbital of C-Cl bond, the HOMO of the nucleophile will donate its electron density to the LUMO of the dichlorocarbene when the orbitals are correctly aligned (i.e. the LUMO came in perpendicular to the HOMO). This addition will be highly favoured as there is no steric concern in here.
With the HOMO of the nucleophile has its largest electron density localised in the endo alkene bond and the LUMO of the dichlorocarbene eletrophile in the largely p-based antibonding orbital of C-Cl bond, the HOMO of the nucleophile will donate its electron density to the LUMO of the dichlorocarbene when the orbitals are correctly aligned (i.e. the LUMO came in perpendicular to the HOMO). This addition will be highly favoured as there is no steric concern in here.
Part 2: Influence of C-Cl bond on the Vibrational Frequency
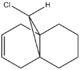
In this section, to study the influence of the C-Cl bond on the vibrational energies of the original structure 12 and 12i as shown in Fig. 1.4h , IR spectra for both of the molecules are modelled.
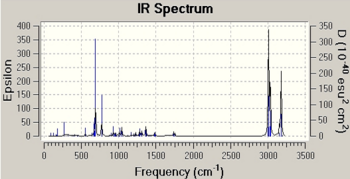
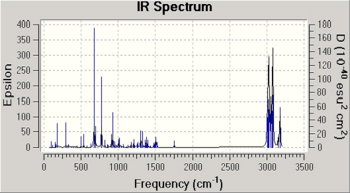
The key bond vibrations are highlighted as follow:
| Molecule | C-Cl stretch/ cm-1 | synC=C stretch/cm-1 | antiC=C stretch/cm-1 |
|---|---|---|---|
| Structure 12 | 770.65 | 1757.48 | 1737.59 |
| Structure 12i | 775.03 | 1753.66 | - |
The C-Cl bond increases in its vibrational energy as it goes from 12 to 12i and this indicates that the C-Cl bond is stronger in 12i than 12. In structure 12, the C-Cl bond is aligned in anti-periplanar (app) fashion to the πC=C,anti orbital, allowing electron density from π double bond to donate into σ*C-Cl orbital and hence weakening the C-Cl bond due to this app stabilisation. However in structure 12i, this app alignment is lost and electron density is now more concentrated on the C-Cl bond. This increase in localised electron density in turn donates into the π orbital of syn C=C bond and stabilised it, hence leading to a weaker bond and a decrease in syn C=C stretching frequency.
.
Mini Project - Investigation on Endo and Exo Camphor using DFT based Molecular Orbital Method
Introduction
Camphor is a waxy, white and aromatic natural product that can be found in the wood of Camphor Laurel, a large evergreen tree. Synthetically, camphor can also be produced from the oil of turpentine. Its aromatic scent is commonly used as an ingredient in cooking, as an embalming fluid for medicinal purposes and in modern days, it is used as a plsticizer for nitrocellulose as a moth repellent and even in fireworks. Further reactions such as oxidation, bromination and Mannich reaction can be carried out on camphor. In this mini project, the Mannich reaction of 3-(hydroxymethylene)-d-camphor is studied with the following reaction scheme:
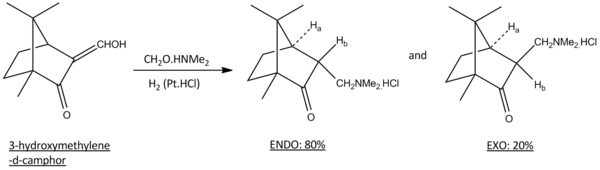
Hine and co-workers first treated 3-(hydroxymethylene)-d-camphor with dimethylamine followed byhydrogenation in the presence of platinum oxide and hydrochloric acid[6]. This results in a very good yield of 90% with 80% exo-isomer and 20% endo isomer. The presence of this diastereomers was proven with NMR results and could be isolated with repeated crystallisation.
To identify and differentiate between isomers, 13C NMR and 1H NMR will be suitable to indentify as the protons and the carbons couples to each other differently. Also, optical rotation will be good to identify stereoisomers as it will rotate the plane polarized light in opposite directions. It is suggested by Natalie and co-workers that the difference in the dihedral angles between Ha and Hb does influence the H coupling in 1NMR[7]. In this example, camphor derivatives were modelled and optimised to predict 13C NMR spectra.
Predict 13C NMR spectra of Camphor derivative isomers
The optimised energies of both diastereomers were first run and tabulated in Table 2i. It is in good agreement with theoretical expectation that the Endo product is thermodynamically stable based on steric considerations similar to that as discussed in 1.1.
| Energy/kcal mol-1 | Exo Product | Endo Product |
|---|---|---|
| Stretch | 2.0363 | 1.9525 |
| Bend | 14.8838 | 14.1463 |
| Stretch-Bend | -0.2020 | -0.1154 |
| Torsion | 15.5082 | 16.0331 |
| Non-1,4 VDW | -0.3975 | -1.9941 |
| 1,4 VDW | 8.8985 | 9.4383 |
| Dipole-Dipole | 0.6232 | -0.1182 |
| Total Energy | 41.9506 | 39.3424 |
The computed camphor derivative is fully optimised at the DFT-mpw1pw91/6-31(d,p) level and a comparison between the literature and the predicted values are tabulated as follow.
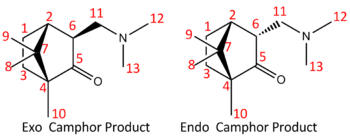
| Corresponding Carbon | Predicted Value/ppm | Lit. Value/ppm | Difference/ppm |
|---|---|---|---|
| 1 | 37.3194 | 31.3 | -6.0194 |
| 2 | 13.7768 | 49.9 | 36.1232 |
| 3 | 16.9504 | 20.6 | 3.6496 |
| 4 | 50.6142 | 56.3 | 5.6858 |
| 5 | 205.378 | 222.6 | 17.222 |
| 6 | 41.1653 | 46.6 | 5.4347 |
| 7 | 37.0318 | 46.4 | 9.3682 |
| 8 | 13.1555 | 19.4 | 6.2445 |
| 9 | 12.8694 | 18.9 | 6.0306 |
| 10 | 11.9053 | 9.3 | -2.6053 |
| 11 | 46.6986 | 59.8 | 7.0143 |
| 12 | 37.1857 | 44.2 | 7.0143 |
| 13 | 31.4493 | 43.8 | 12.3507 |
| Corresponding Carbon | Predicted Value/ppm | Lit. Value/ppm | Difference/ppm |
|---|---|---|---|
| 1 | 21.6696 | 29.2 | 7.5304 |
| 2 | 40.2072 | 50.2 | 9.9929 |
| 3 | 16.2684 | 28.4 | 12.1316 |
| 4 | 50.9022 | 58.4 | 7.4978 |
| 5 | 204.854 | 222.9 | 18.046 |
| 6 | 45.0371 | 48.6 | 3.5629 |
| 7 | 37.9855 | 47.7 | 9.7145 |
| 8 | 15.3405 | 21.1 | 5.7595 |
| 9 | 13.5321 | 19.8 | 6.2679 |
| 10 | 12.3594 | 9.3 | -3.0593 |
| 11 | 53.372 | 56.3 | 2.9328 |
| 12 | 37.5169 | 43.8 | 6.2831 |
| 13 | 33.6605 | 43.5 | 9.8395 |
The results were not in very good agreement with that of the literatures but it is generally out of literature by a consistent range of ~10ppm. This error may be attributed to wrong setting in setting the reference for the NMR peaks and the limitations of this calculation method itself. The largest deviation between the literature and the predicted carbon shift corresponds to the carbonyl carbon. This shift is abnormally high due to its high deshielding nature caused by oxygen and its rigid structure. Also, the larger the chemical shift, it is likely to incur a greater inaccuracy in the calculation.
Reference
William L. Jorgensen, Dongchul Lim, James F. Blake,J. Am. Chem. Soc., 1993, 115 (7), pp 2936–2942.DOI:10.1021/ja00060a048
Brian Halton, Sarah G. G. Russell,J. Org. Chem., 1991, 56 (19), pp 5553–5556.DOI:10.1021/jo00019a015
- ↑ Stanley J. Cristol, Wolfgang K. Seifert, S. Barney Soloway, Bridged Polycyclic Compounds,1960,82(9),2351-2356.DOI:10.1021/ja01494a060
- ↑ D. Skala, J. Hanika, Petroleum and Coal, 2003, Vol. 45, 105-108.
- ↑ 3.0 3.1 A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838 DOI:10.1021/jo00356a016 Cite error: Invalid
<ref>tag; name "jo00356a016" defined multiple times with different content - ↑ 4.0 4.1 W.F. Maier, P.V.R.Schleyer, J.Am.Chem. Rev., 1976,7, 31DOI:10.1021/ja00398a003
- ↑ Alan B. McEwen, Paul v. R. Schleyer,J. Am. Chem. Soc., 1986, 108 (14), pp 3951–3960Template:DOI: 10.1021/ja00274a016
- ↑ Hine. J.,W.-S.,J.Am.Chem.Soc.9 75, 97,3550-3551
- ↑ Natalie L. McClure, Gui Yuan Dai, Harry S.Mosher,J.Org.Chem., 1988, 53(11), pp2617-2620. DOI:10.1021/jo00246a042