Carbon Dioxide
Carbon dioxide.pdb |
General
| Carbon Dioxide | |
|---|---|
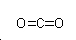
| |
| General | |
| Systematic name | Carbon Dioxide |
| Other names | Carbonic acid gas; carbonic anhydride; dry ice (in solid state) |
| Molecular formula | CO2 |
| SMILES | O=C=O |
| Molar mass | 44.0095(14) g/mol |
| Appearance | Colourless gas |
| CAS number | 124-38-9 |
| Properties | |
| Density & phase | 1,600 kg/m in solid state; 1.98 kg/m³ in gaseous state |
| Solubility in water | 1.45 kg/m³ |
| Melting point | 194.65 K |
| Boiling point | 195 K |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | 0.07 cP at 195 °C9 |
| Structure | |
| Molecular shape | Linear |
| Coordination geometry |
Not available |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | {{{DM}}} D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Asphyxiant in high concentrations |
| NFPA 704 | None |
| Flash point | None |
| R/S statement | R: {{{R-S}}} S: ? |
| RTECS number | {{{RTECS}}} |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Carbon dioxide is a chemical compound made up from two oxygen atoms covalently bonded to a single carbon atom. At room temperature and pressure, carbon dioxide exists as a gas. The chemical formula for carbon dioxide is CO2. Most commonly, carbon dioxide is used up in one of the most important processes of life, photosynthesis. It is used to form glucose, water vapour and oxygen as a by-product, which is the same oxygen available to humans for respiration, where the opposite gas transfer takes place, and carbon dioxide is released, while oxygen is taken up. Additional carbon dioxide is also created by the combustion of fossil fuels or vegetable matter, among other chemical processes. Carbon dioxide is referred to as a green house gas, as it traps radiation from the sun to keep heat from fully escaping. Due to the fact that it absorbs in the infra red region of light, and because of its atmospheric life time, it plays a major role in the carbon cycle.
Physical and Chemical Properties
Carbon dioxide is a colourless and odourless gas. At standard temperature and pressure, carbon dioxide has a density of 1.98kg/m³, which is about one and half times greater than that of air. The VSEPR theory predicts that carbon is bonded from two double bonds, one from each oxygen leaving two sets of lone pairs on each of the oxygen, which best arranges best itself to a linear shape due to electron repulsion. The molecule has no electrical dipole, and because it’s fully oxidised, it is reasonably reactive and non flammable, but can be used as support in the combustion of certain metals like magnesium.
Carbon dioxide has some interesting effects in the human body. It has been proven that if carbon dioxide is inhaled at concentrations higher than the normal atmospheric levels, it can produce s stinging sensation in the nose and throat, and a sour taste in the mouth. This is the result of carbon dioxide dissolving the mucous membranes and saliva, which forms a very solution of carbonic acid. This sensation also occurs at an attempt to stifle a burp after drinking a carbonated drink.
At a low temperature of about 194.64 K, carbon dioxide changes from a solid to a gaseous phase through the process of sublimation. It’s commonly referred to as dry ice, and was first observed by a French chemist called Charles Thilorier in 1825. There are however other forms of solid carbon dioxide are known today. One of them is the amorphous glass like form which can be formed under very strong pressure. This form of glass is known as carbonia, which was produced by cooling heated carbon dioxide at extreme pressure (of about 400,000 atm) in a diamond anvil. The research established that carbon dioxide could exist in a glass form just like other elements in Group 14, such as silicon and germanium. However due to its instability, the carbon dioxide glass sublimed back to gas when exposed at standard conditions.
Liquid carbon dioxide only forms at pressures above 5.1 atm. The triple point of carbon dioxide is approximately 518 kPa at 218.55 K and the critical point is 7,821 kPa at 306.25 K (data from the phase diagram).
Uses:
Carbon dioxide is widely used in many industries, and has important roles in the food, oil, and chemical industry.
Industrial production:
Carbon dioxide in the atmosphere:


