COOKIESANDCREAM
Matthew Wilfred Ho
The Hydrogenation of Cyclopentadiene Dimer
The Diels-Alder reaction is a [4+2] cycloaddition between a diene and a dienophile, an alkene that usually is, but not necessarily, conjugated with an electron-withdrawing group. In the case of cyclopentadiene, it can behave both as the diene and the dienophile, thus dimerizing to form dicyclopentadiene in either of two stereoisomers: the exo- (1) or endo-(2) form.
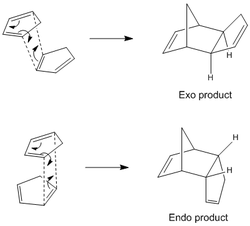
An analysis of the relative energies of the two products was performed. The structures of (1) and (2) were optimized with the Allinger MM2 force field, giving the following results:
| Product | % difference (endo-exo) | |||
|---|---|---|---|---|
| Molecule number | 1 (exo) | 2 (endo) | ||
| Stretch | 1.2857 | 1.2521 | -1.6 | |
| Bend | 20.5808 | 20.8472 | 12.6 | |
| Stretch-bend | -0.8384 | -0.8356 | 0.1 | |
| Torsion | 7.6542 | 9.5108 | 87.5 | |
| Non-1,4 VDW | -1.14166 | -1.5430 | -6.0 | |
| 1,4 VDW | 4.2334 | 4.3185 | 4.0 | |
| Dipole/dipole | 0.3775 | 0.4475 | 3.3 | |
| Total | 31.8765 | 33.9975 | ||
The tabulated results show the Torsion term to be the chief contribution in destabilizing the endo- product (2), which when summed together with all the other terms results in delta E = -2.1 kcal/mol favouring the exo- product (1) over the endo- product (2), making (1) the thermodynamic product.
However, it is well documented in the literature that the endo- product (2) predominates instead. This suggests the reaction is under kinetic control, with a reaction profile of this form:
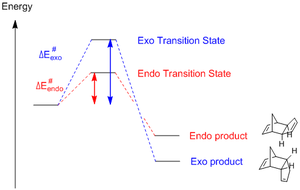
The inverted stabilities of the transition states to their respective products can be rationalised by Molecular Orbital interactions in the transition state:
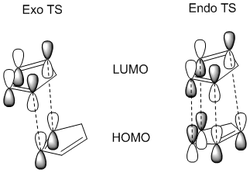
The endo transition state benefits from 4 sets of stabilizing MO interaction, whereas the exo transition has only 2; this renders the endo TS stabler and lowers the activation energy required for the formation of the endo product (2) relative to (1), making (2) the kinetic product.
Given the material fact that the endo product (2) predominates in the dimerization of cyclopentadiene, we can come to the conclusion that the reaction is under kinetic control.
The endo- product (2) was further investigated in a hydrogenation reaction that gives two possible dihydro derivatives (3) and (4):
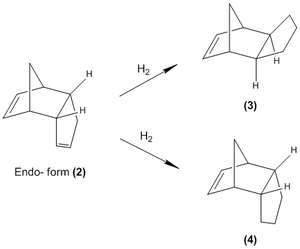
Again using the Allinger MM2 force field, the structures of (3) and (4) were optimized giving the following results:
| Product | % difference (endo-exo) | |||
|---|---|---|---|---|
| Molecule number | 3 | 4 | ||
| Stretch | 1.2782 | 1.0973 | 4.0 | |
| Bend | 19.8640 | 14.5245 | 117.8 | |
| Stretch-bend | -0.8349 | -0.5494 | -6.3 | |
| Torsion | 10.8073 | 12.4982 | -37.3 | |
| Non-1,4 VDW | -1.2236 | -1.0702 | -3.4 | |
| 1,4 VDW | 5.6320 | 4.5111 | 24.7 | |
| Dipole/dipole | 0.3775 | 0.4475 | 3.3 | |
| Total | 35.6850 | 31.1521 | ||
For the hydrogenated products, the product (4) was found to be stabler by delta E = -4.5 kcal/mol. The greatest contribution by for to the stability of (4) is its lower bend strain, accounting for 117.8% of its energy stabilized relative to (3); as a point of comparison, with (1) and (2) the bend strain only contributed 12.6%.
From (2) to (4), the delta E(bend) = -6.3kcal/mol; from (2) to (3), the delta E(bend) = -1.0 kcal/mol. The results from the calculations indicate that the hydrogenation of the endo- form (2) to (4) is more thermodynamically favourable than that of (2) to (3), mainly due to a greater relief in bend strain.
This is borne out by the structures obtained via MM2 optimization. Going from (2) to (3), the dihedral angle of the bond prior to hydrogenation is 113 degree; while from (2) to (4), the dihedral angle of its respective bond is 108 degree. Keeping in mind that for C=C double bonds a value of ~120 degree is best, the bend strain relieved in hydrogenating the 108 degree bond when forming (4), is greater than that of the 113 degree bond when forming (3). This agrees well our prior calculation.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Taxol, or Paclitaxel, is an anti cancer drug used in the treatment for breast, ovarian and lung cancers. The total synthesis proposed by Paquette involves a key intermediate that exists as 2 interconverting but isolable atropisomers, (9) and (10):
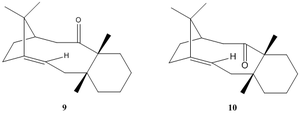
The Allinger MM2 and MMFF94 force fields were used to optimize the structures of (9) and (10), yielding up two conformers with each: the suffixes “chair” and “ twist coat” denote the conformation of the cyclohexane ring.
| Isomer 9 | Isomer 10 | |||
|---|---|---|---|---|
| Molecule number | Chair | Twist boat | Chair | Twist boat |
| Stretch | 2.7845 | 2.9482 | 2.6204 | 2.7115 |
| Bend | 16.5412 | 17.2124 | 11.3388 | 11.7417 |
| Stretch-bend | 0.4304 | 0.5015 | 0.3432 | 0.3237 |
| Torsion | 18.2514 | 21.2834 | 19.6719 | 21.8662 |
| Non-1,4 VDW | -1.5527 | -1.4164 | -2.1615 | -2.0968 |
| 1,4 VDW | 13.1096 | 14.5057 | 12.8724 | 13.9188 |
| Dipole/Dipole | -1.7248 | -1.7304 | -2.0023 | -2.0316 |
| Total Energy MM2 | 47.8395 | 53.3043 | 42.6828 | 46.4335 |
| Total Energy MMFF94 | 70.5411 | 76.2722 | 60.5445 | 66.2783 |
For the purposes of this analysis the energies of the stablest conformers are compared: namely 9 chair and 10 chair. The data shows clearly that the 10 chair isomer is stabler than the 9 chair, with delta E = -5.2 kcal/mol stabilization, with the Bend strain term contributing most to the stability of the 10 chair (delta E(bend) = -5.2kcal/mol). The result from MMFF94 optimization confirms the stability of 10 chair, with a total energy lower than that of 9 chair by 10 kcal/mol.
The greater bend strain experienced by (9) can be rationalised by the steric crowding on the upper face of the molecule, caused by the proximity of the bridgehead methyl groups and the upward-pointing carbonyl group; an unfavourable interaction that is missing in (10).
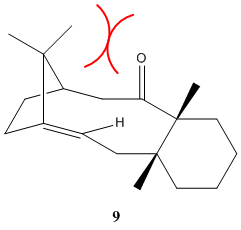
The overall conclusion is that the atropisomer (10) is stabler than (9), making it the thermodynamic product.
The compounds (9) and (10) are also of interest in that they are hyperstable olefins: in a 10-ring macrocyclic such as these two, due to the bridgehead alkene the unsaturated compound actually has lower steric strain than their respective hydrogenated products, which explains the unusually low reactivity of the alkenes. This is illustrated by comparing (9) and (9) hydrogenated:
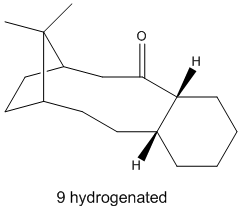
| Product | ||
|---|---|---|
| Molecule number | 9 Chair | 9 Hydrogenated |
| Stretch | 2.7845 | 3.0527 |
| Bend | 16.5412 | 16.4217 |
| Stretch-bend | 0.4304 | 0.6226 |
| Torsion | 18.2514 | 24.9774 |
| Non-1,4 VDW | -1.5527 | -0.2916 |
| 1,4 VDW | 13.1096 | 16.5210 |
| Dipole/dipole | -1.7248 | 0.0000 |
| Total | 47.8395 | 61.3007 |
The item to emphasize here is the torsion term: the hydrogenated form is greatly destabilized by its increased torsional strain. This can be quantified as “olefin strain”, or the difference in torsional energy between the hydrogenated compound and its alkene counterpart: E = 24.9774-18.2514 = -6.7260 kcal/mol favouring the alkene. It is this negative value that defines hyperstable alkenes.
Regioselective Addition of Dichlorocarbene
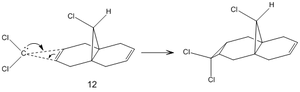
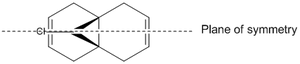
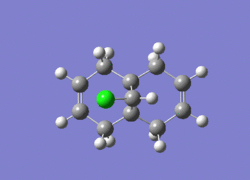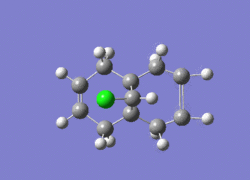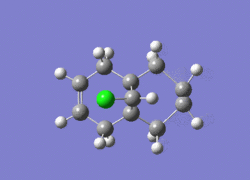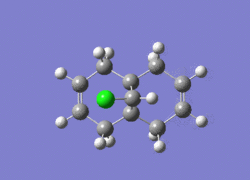
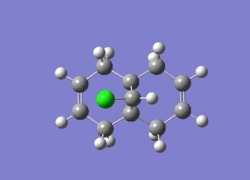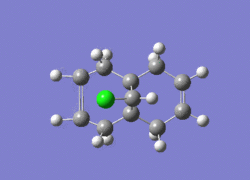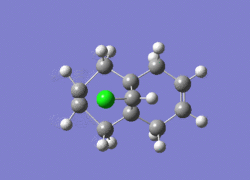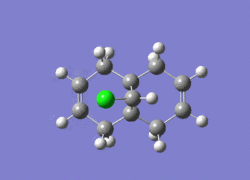
Dichlorocarbene is a very reactive electrophile that reacts with (12) in a cycloaddition resulting in a cyclopropane group, with good regioselectivity shown above with attack on the endo alkene. To model the reaction correctly, the previously applied molecular mechanics methods cannot be used as they do not account for molecular orbital interactions.
The approach taken was:
1. Preliminarily optimize structure with MM2
2. Compare structures from further optimization with MOPAC/PM6 and MOPAC/RM1
3. Choose MOPAC/RM1 for better Cs symmetry
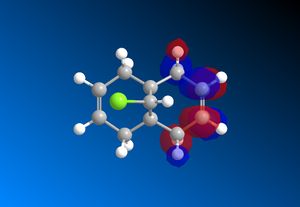
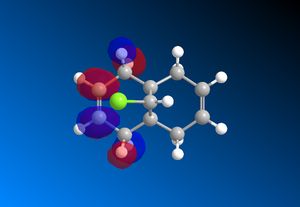
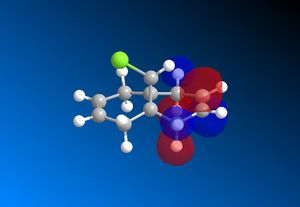
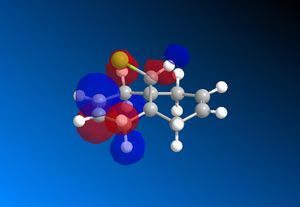
The RM1 method was picked over PM6, because the PM6 optimized structures did not give molecular orbitals that were symmetrical as they should be with (12), which has Cs symmetry along the middle:
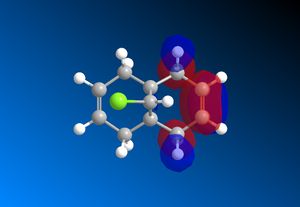
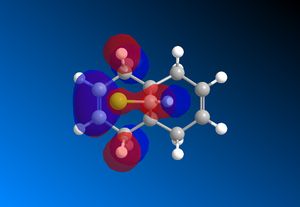
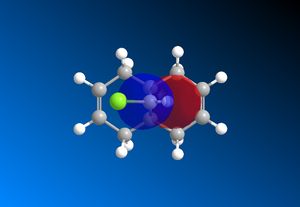
The computed MO’s with MOPAC/RM1 are as shown:
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
 |
 |
 |
 |
 |
 |
 |
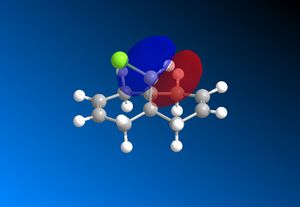 |
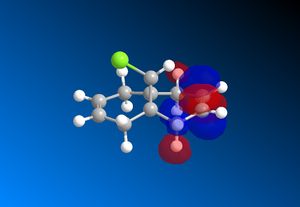 |
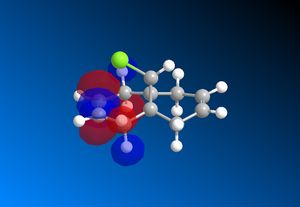 |
The IR spectrum of (12) was also predicted using MOPAC/RM1:
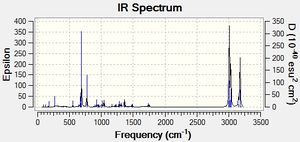
Additionally, the exo-hydrogenation product, (13) was also analyzed:
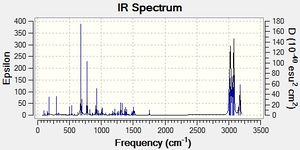
| Molecule | C-Cl | C=C Exo | C=C Endo |
|---|---|---|---|
| 12
|
770.83cm-1 |
1757.45cm-1 |
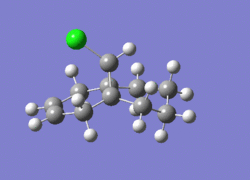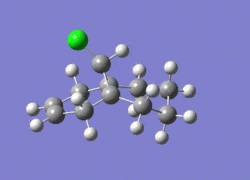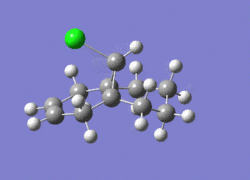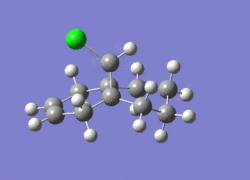
1737.06cm-1 |
| 13
|
774.95cm-1 |
1758.05cm-1 |
N/A |
The C-Cl bond increases in strength from (12) to (13), since hydrogenation removes the exo- alkene that previously contributed electron density to the sigma* C-Cl antibonding orbital, resulting in a stronger stretch. The removal of the exo C=C bond results in the loss of the exo C=C stretch.
Monosaccharide chemistry: glycosidation
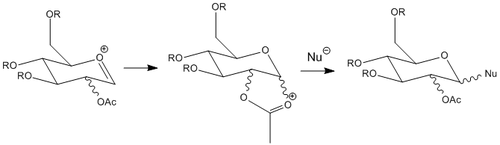
The stereochemistry of the glycosidation reaction is usually determined by the steric bulk of the constituents and the anomeric effect, but can be altered by the presence of an –OAc group adjacent to the anomeric carbon, also known as the neighbouring group participation. To model its effects, methyl groups were used as the R group to minimize computation time. Both MM2 and MOPAC/PM6 were used to optimize the structures of A and B, where the -OAc group occupies the equatorial and axial positions respectively; C and D are the respective bicyclics formed by the attack of the carbonyl group on -OAc on the anomeric carbon:
| Molecule | A | A\' | B | B\' | C | C\' | D | D\' |
|---|---|---|---|---|---|---|---|---|
| Stretch | 2.2433 | 2.3645 | 2.3118 | 2.3313 | 1.7464 | 2.7124 | 1.8364 | 2.6609 |
| Bend | 10.7845 | 11.1359 | 11.0779 | 14.0753 | 16.0951 | 17.4666 | 13.3750 | 19.1784 |
| Stretch-bend | 0.8088 | 0.9071 | 0.9196 | 0.9089 | 0.6987 | 0.7956 | 0.6230 | 0.7322 |
| Torsion | 1.7143 | 3.2320 | 2.2578 | 0.1879 | 7.7931 | 8.1660 | 6.1162 | 6.9579 |
| Non-1,4 VDW | -1.8723 | -2.5262 | -1.0457 | -0.9662 | -4.0463 | -2.4355 | -3.8713 | -2.3769 |
| 1,4 VDW | 19.2528 | 19.2621 | 19.8167 | 19.3787 | 17.6967 | 19.3976 | 18.0489 | 18.8441 |
| Charge/Dipole | -15.6640 | -10.1861 | -24.9311 | -15.5976 | -5.0476 | -0.3318 | 0.9059 | -2.8813 |
| Dipole/dipole | 4.1836 | 4.3244 | 6.7924 | 6.6785 | -0.4045 | -1.7451 | -0.3195 | -1.5584 |
| Total (MM2) | 21.4512 | 28.5136 | 17.1995 | 26.9966 | 34.5316 | 44.0259 | 36.7147 | 41.5569 |
| Heat of formation (PM6) | -91.66208 | -77.39780 | -83.49323 | -68.23351 | -91.66308 | -66.88323 | -84.07642 | -67.00296 |
There is a special point to note in the Heat of Formation values obtained by MOPAC/PM6 optimization. There are two pairs of molecules that have identical (or near identical) heat of formation values: A and C, B and D. The consequence of this is that A and C will be in equilibrium, and B and D will be in equilibrium as well; however since entropy is not accounted for in this analysis the position of the equilibrium cannot be commented upon.
Mini-project: Assigning regioisomers in "Click Chemistry"
Rolf Huisgen's dipolar cycloaddition of organic azides and alkynes reacts to form a product in two isomers: 1,4- and 1,5-disubstituted 1,2,3-triazoles. Recent advances with the use of of a Cu(I) catalyst has sped the reaction up sufficiently that it is now regarded a "click reaction".
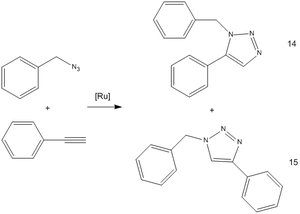
In this particular reaction, a Ruthenium based catalyst is used instead and isomers 14 and 15 are formed as products, with the 1,5-isomer (14) being the major product. The structures of both 14 and 15 were optimized using MM2 and MOPAC/AM1 for NMR spectra prediction with SCAN, and tabulated thus:
| Product | ||
|---|---|---|
| Molecule number | 14 | 15 |
| Stretch | 1.1061 | 0.7733 |
| Bend | 18.3004 | 13.1250 |
| Stretch-bend | 0.1075 | -0.0301 |
| Torsion | -15.7267 | -14.8800 |
| Non-1,4 VDW | 0.7258 | -1.6492 |
| 1,4 VDW | 14.3724 | 14.7102 |
| Dipole/dipole | -1.6628 | -1.3999 |
| Total | 17.2227 | 10.6494 |
| Heat of formation (PM6) | 143.44864 | 142.13144 |
NMR Spectra
The structure was then optimized through SCAN using the method mpw1pw91/6-31g(d,p) opt(maxcycle=25); afterwards the C13 NMR was predicted using mpw1pw91/6-31(d,p) NMR scrf(cpcm,solvent=chloroform). Carbon-13 NMRs are more reliably predicted in computational chemistry than proton NMRs, and is thus chosen for this investigation.
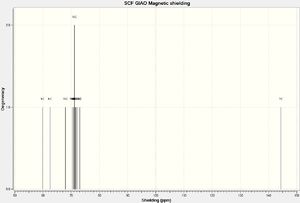
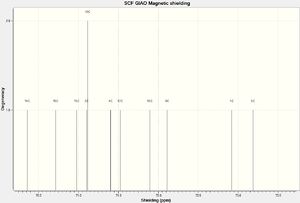
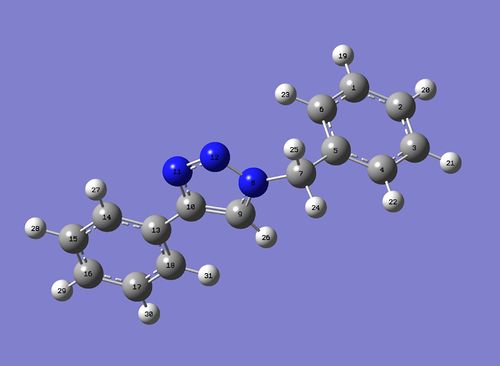
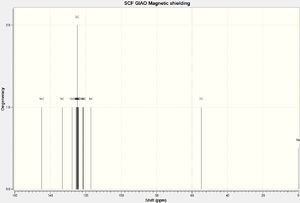
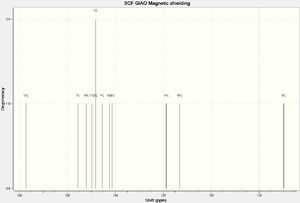
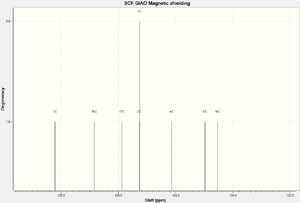
IR Spectra
The IR Spectrum was also predicted:
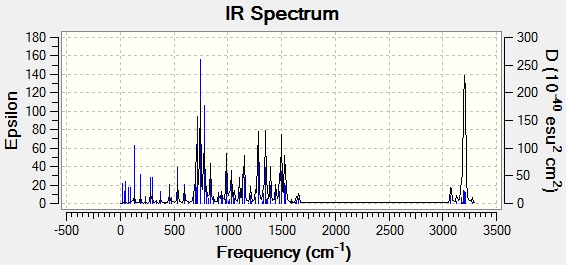
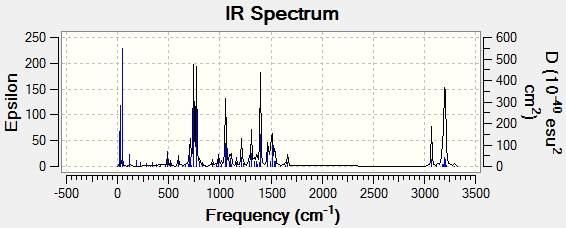
Conclusion
The NMR and IR spectra of the isomers 14 and 15 were predicted.
