CID 01398966CK
NH3 Molecule
N-H bond distance: 1.01798 Å
H-N-H bond angle: 105.741°
NH3 optimisation
File Name nh3 optimisation File Type .log Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -56.55776873 a.u. RMS Gradient Norm 0.00000485 a.u. Imaginary Freq 0 Dipole Moment 1.8466 Debye Point Group C3V Job cpu time: 0 days 0 hours 0 minutes 54.0 seconds.
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986279D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
All relevant information available Here
NH3 molecule |
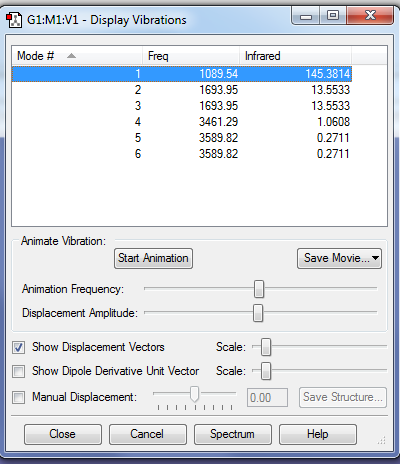
Vibrations
Using the 3N-6 rule, 6 vibrational modes are expected to arise. Modes of the same frequency are degenerate. Modes 4,5 and 6 are stretching as they vibrate at much higher frequencies compared to modes 1,2 and 3 which are bending. This deduction can be done since stretching modes require higher energies to form (hence higher frequencies) Mode 4 being highly symmetric. Mode 1 is known as the "umbrella" mode. 2 peaks are present in the ammonia IR spectrum. Peaks are present as the molecule has vibrations which cause a change in the dipole moment and hence are IR active. The first peak occurs due to Mode 1. The second peak occurs due to modes 2 and 3 which are degenerate bends and appear as one peak.
These 2 peaks almost correspond to the actual IR spectrum even though broadness and relative intensities are not exactly reflected by the approximation.
Nitrogen holds a negative charge of value -1.125. Hydrogen holds a positive charge of value +0.375. These values are consistent with expected values as Nitrogen is much more electronegative than hydrogen, hence the most of the negative charge resides on it.
N2 Molecule
N-N Bond distance : 1.10550 Å
N2 optimisation
CK 01398966 File Name ck2917_n2_optimisation File Type .log Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -109.52412868 a.u. RMS Gradient Norm 0.00000060 a.u. Imaginary Freq 0 Dipole Moment 0.0000 Debye Point Group D*H Job cpu time: 0 days 0 hours 0 minutes 58.0 seconds.
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401085D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
All relevant information available Here
N2 molecule |
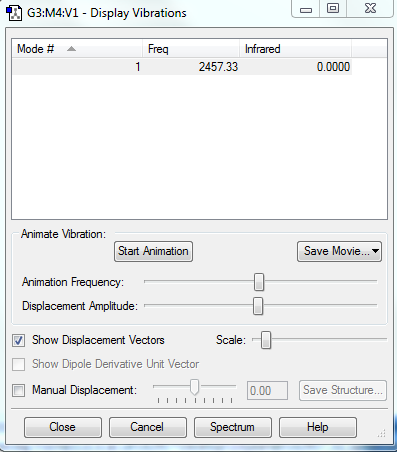
Vibrations
Using the 3N-5 rule, 1 vribrational mode is expected to be shown. Only one symmetric stretching mode is present. IR inactive as no dipole moment is created.
H2 Molecule
H-H bond length: 0.74279 Å
H2 optimisation
ck2917_h2_optimisation File Name ck2917_h2_optimisation File Type .log Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -1.17853936 a.u. RMS Gradient Norm 0.00000017 a.u. Imaginary Freq 0 Dipole Moment 0.0000 Debye Point Group D*H Job cpu time: 0 days 0 hours 0 minutes 42.0 seconds.
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
All relevant information available Here
H2 molecule |
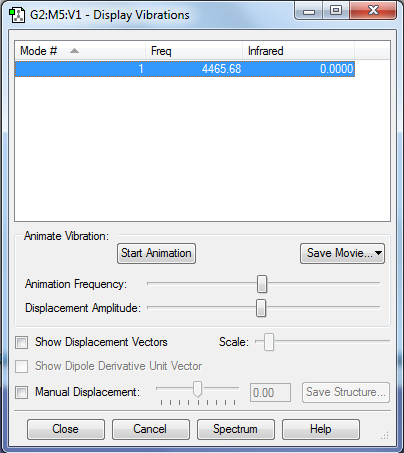
Vibrations
Using the 3N-5 rule, 1 vribrational mode is expected to be shown. Only one symmetric stretching mode is present. IR inactive as no dipole moment is created.
Energy calculations
E(NH3)= -56.55776873 a.u
2*E(NH3)= -113.11553746 a.u
E(N2)= -109.52412868 a.u
E(H2)= -1.17853936 a.u
3*E(H2)= -3.53561808 a.u
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u
= -0.0557907 x 2625.5 = -146.47848285 kJ/mol.
Change in energy (ΔE) which is Eproducts-Ereactants will give us the enthalpy. Due to a negative energy change, ammonia (product) is more stable than hydrogen/nitrogen (reactants). This energy change is not as accurate as the actual value as a result of the quantum mechanical approximation we are running which causes some shifts in wavenumber values as a result energy values vary.
HF2- Molecule
H-F bond length: 1.15056 Å
HF2- Optimisation
ck2917_hf2-_optimisation File Name ck2917_hf2-_optimisation File Type .log Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge -1 Spin Singlet E(RB3LYP) -200.28998965 a.u. RMS Gradient Norm 0.00000297 a.u. Imaginary Freq 0 Dipole Moment 0.0000 Debye Point Group D*H Job cpu time: 0 days 0 hours 1 minutes 8.0 seconds.
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000023 0.001800 YES
RMS Displacement 0.000016 0.001200 YES
Predicted change in Energy=-1.460268D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1506 -DE/DX = 0.0 !
! R2 R(2,3) 1.1506 -DE/DX = 0.0 !
! A1 L(1,2,3,-2,-1) 180.0 -DE/DX = 0.0 !
! A2 L(1,2,3,-3,-2) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
All relevant information available Here
NH3 molecule |
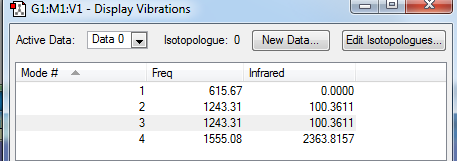
Vibrations
From the 3N-6 rule, 3 vibration modes are expected which is consistent with the results obtained. In the IR spectrum only 2 peaks are seen. Mode 1 is a symmetric stretch which does not produce a dipole moment and so it is IR inactive. Modes 2 and 3 are degenerate and so only one peak is formed. Mode 4 is responsible for the most intense and broad peak in the spectrum as a result of the large dipole moment created by this vibration.
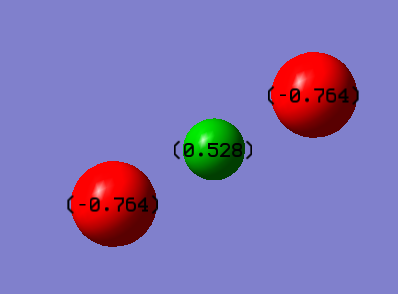
Fluorine holds a negative charge of -0.764 and hydrogen holds a positive charge of +0.528. This is consistent with expectations and theory as fluorine as much more electronegative that hydrogen as a result most of the negative charge resides on the fluorine atoms.
H2 + 2 F2 = 2 HF2
Energy of F2 calculated using GaussView.
E(HF2-)= -200.28998965 a.u.
2*E(HF2-)= -400.5799793 a.u.
E(F2)= -199.49825218 a.u.
2*E(F2)= -398.99650436 a.u.
E(H2)= -1.17853936 a.u
ΔE=2*E(HF2-)-[E(H2)+2*E(f2)]=
= -0.40493558 x 2625.5 = -1063.15836529 kJ/mol.
Molecular Orbitals
The first two molecular orbitals are very low in energy as a result of fluorine being so much more electronegative than hydrogen. The AO's responsible for them are the two 1s orbitals of fluorine in and out of phase.
MO:3 |
This is similar to the case mentioned above. The overlap between the two 2s AO's of fluorine create this MO (σg). However due to the large energy gap between it and the hydrogen 1s, these are non-bonding pair of electrons.
MO:5 |
This is a bonding molecular orbital, σg, as a result of the 1s of the hydrogen combining with one of the 2p's of the fluorine, say 2pz (σg). This bonding pair of electrons is delocalised over three atoms. A higher contribution from fluorine to this orbital is seen from its shape as a result of fluorine 2p orbitals are closer in energy to the MO than the hydrogen 1s.
MO:6 |
By linearly combining the orbitals in order, the three different p-orbitals combine with each other accordingly. In this case, two other degenerate AO's of fluorine combined, say 2px (πu). This is also a non-bonding pair of electrons.
MO:8 |
This is the antibonding molecular orbital of the previous one (πg).
MO:10 |
This is the result of the same combination of AO's as MO:5 but in this case, the 2pz orbitals are out of phase (σu). This is the HOMO of the molecule.
MO:11 |
This is the antibonding molecular orbital of MO:6 (πg*). It also is the LUMO of this molecule.