CHEMIMM2P
NH3 Molecule
| Molecule | NH3 |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) | -56.55776873(a.u.) |
| RMS Gradient | 0.00000485(a.u.) |
| Point Group | C3V |
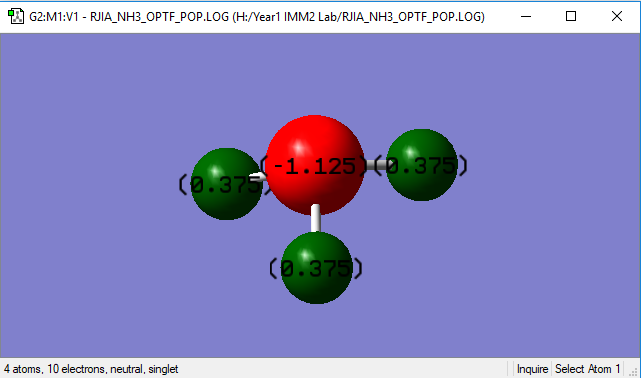
| N-H Bond Length | 1.01798(Å) |
| N-H-N Bond Angle | 105.741o |
NH3 Molecule |
[[1]] NH3 Log File
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986287D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
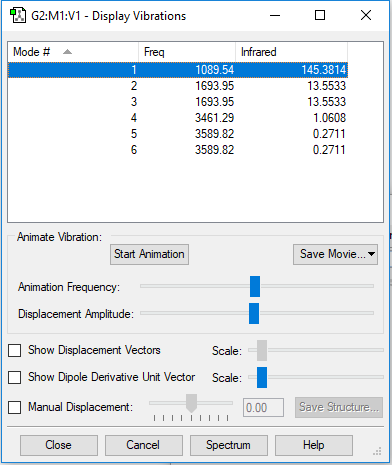
| Wavenumber (cm-1) | 1089 | 1693 | 1693 | 3461 | 3589 | 3589 |
|---|---|---|---|---|---|---|
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity (arbitary unit) | 145 | 13 | 13 | 1 | 0 | 0 |
Answer the following questions in your wiki refer to the vibrations by their wavenumber not their order number!: how many modes do you expect from the 3N-6 rule? Answer : 3*4 - 6 = 6, 6 modes. which modes are degenerate (ie have the same energy)? Answer : Wavenumber 1693 and 1693 are degenerate (Mode 2 and 3). Wavenumber 3589 and 3589 are degenerate (Mode 5 and 6). which modes are "bending" vibrations and which are "bond stretch" vibrations? Answer : Bending vibration: 1089, 1693 and 1693 (Mode 1, 2, 3). Bond stretch vibration : 3461, 3589, 3589(Mode 4, 5, 6). which mode is highly symmetric? Answer : 3461 (Mode 4) one mode is known as the "umbrella" mode, which one is this? Answer : 1089 (Mode 1) how many bands would you expect to see in an experimental spectrum of gaseous ammonia? Answer : two bands
Nitrogen is more electron-negative than hydrogen, which we should expect a negative charge on N atom and positive charge on H atoms.
H2 and N2 Molecules
H2 Molecule
| Molecule | H2 |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) | -1.17853936(a.u.) |
| RMS Gradient | 0.00000017(a.u.) |
| Point Group | D*H |
| H-H Bond Length | 0.74279(Å) |
H2 Molecule |
[[2]] H2 Log File
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
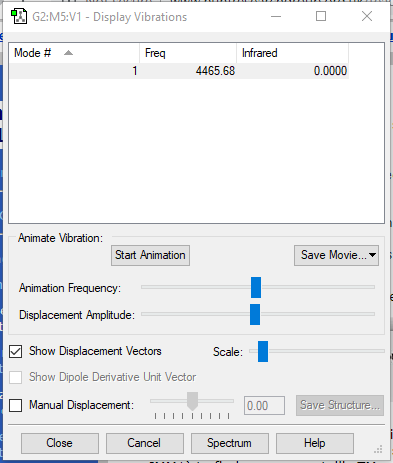
| Wavenumber (cm-1) | 4465 |
|---|---|
| Symmetry | SGG |
| Intensity (arbitary unit) | 0 |
The electron-negativity of Hydrogen atoms are identical, thus it will not have a intermolecular charge within the H2 molecule as expected.
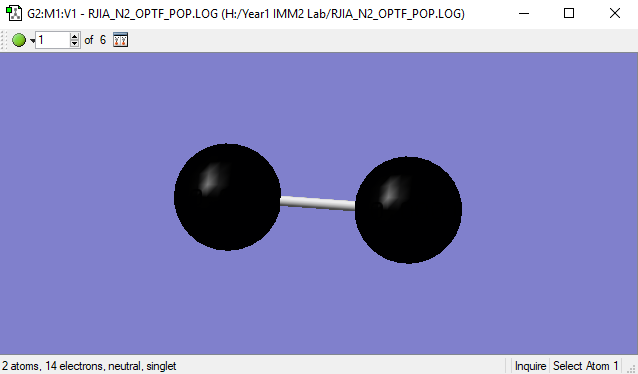
N2 Molecule
| Molecule | N2 |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) | -109.52412868(a.u.) |
| RMS Gradient | 0.00000001(a.u.) |
| Point Group | D*H |
| N-N Bond Length | 1.1055(Å) |
N2 Molecule |
[[3]] N2 Log File
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-9.720915D-17
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
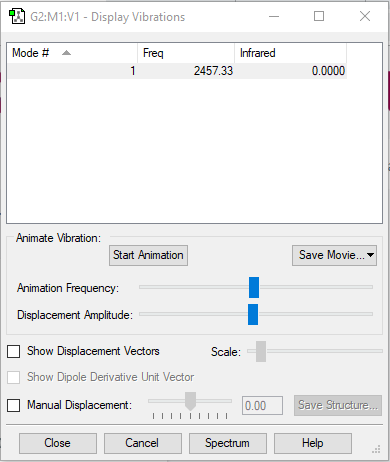
| Wavenumber (cm-1) | 2457 |
|---|---|
| Symmetry | SGG |
| Intensity (arbitary unit) | 0 |
The electron-negativity for both Nitrogen atoms are identical, thus it is expected to not have an intermolecular charge.
Molecule Identifier : VECXEJ Molecule Name : tris(μ-N-(3,5-dimethylphenyl)di(propan-2-yl)phosphanaminido)-(diphenylmethanone)-dinitrogen-titanium-cobalt pentane solvate N-N bond length : 1.101 Å The bond length for my computational result and actual result are very much identical. In the molecule I found, nitrogen had only involved as a ligand, there was no other atoms to have an influence on nitrogen's intermolecular bonding thus it should be very close to the theoretical result. In fact, computational result is calculated based on the theory, and it explains why they have such similar bond length.
Haber Process
E(NH3)= -56.5577687 au 2*E(NH3)= -113.1155375 au E(N2)= -109.5241287 au E(H2)= -1.17853936 au 3*E(H2)= -3.5356181 au ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 au -0.0557907*2625.5 = -146.48 kJ/mol As energy is being released from the reaction, product is in lower energy state than reactants, thus ammonia product is more stable.
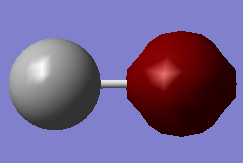
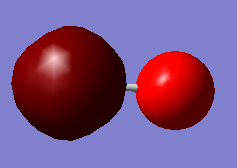
CO Molecule
CO Molecule
| Molecule | CO |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) | -113.30945314(a.u.) |
| RMS Gradient | 0.00000002(a.u.) |
| Point Group | C*V |
| C-O Bond Length | 1.13794(Å) |
CO Molecule |
[[5]] CO Log File
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-6.433359D-16
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1379 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
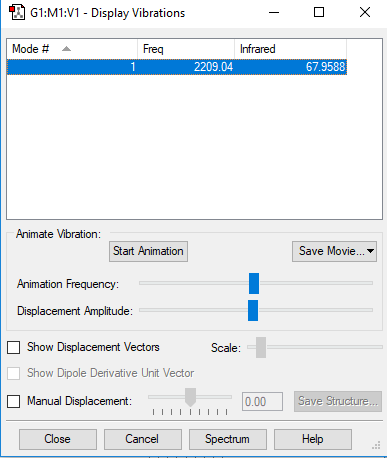
| Wavenumber (cm-1) | 2209 |
|---|---|
| Symmetry | SG |
| Intensity (arbitary unit) | 67 |
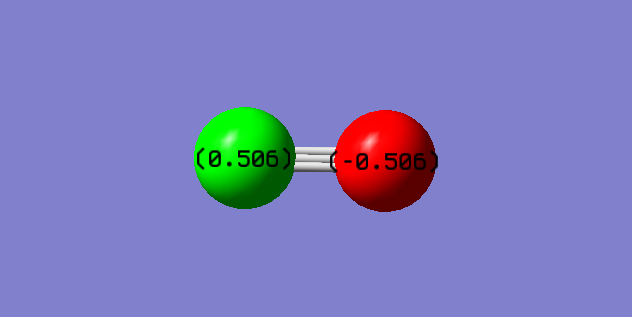
Oxygen (Red atom) is more electro-negative than Carbon (Green), thus it is expect to have a δ- on oxygen and a δ+ on Carbon which is correctly shown above.
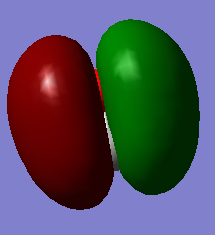
CO Molecular Oribital
This is the the deepest molecular orbital of carbon monoxide, occupied, it has energy -19.25805 au. This is the 1σ orbital formed from the 1s atomic orbital. Since it has such high energy, it doesn't participate in any reaction.
This is the second deepest molecular orbital of carbon monoxide, occupied, it has energy -10.30433 au. This is the 1σ* orbital formed from the 1s atomic orbital. This molecular orbital doesn't participate in any reaction as well.
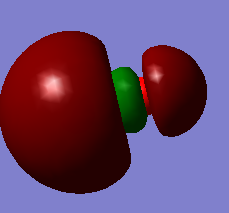
This is the 2σ orbital formed from 2s atomic orbital, occupied, energy level -1.15790
This is the 1π Molecular orbital, occupied, formed from 2px and 2py atomic orbital. It has energy level -0.46743 au.
This is the HOMO (Highest Occupied Molecular Orbital), 3σ orbital formed from 2pz atomic orbital. It has the lowest energy of -0.37145 au.
Comparison of bond length
The actual bond length between Carbon and Oxygen in carbon monoxide is 1.128Å [1], compare to our calculated bond length 1.13794Å, there is a slight difference. This is most likely caused by the ionic character in carbon monoxide molecule that attract the two particles closer to each other.
- ↑ Gilliam, O. R.; Johnson, C. M.; Gordy, W. (1950). "Microwave Spectroscopy in the Region from Two to Three Millimeters". Physical Review. 78 (2): 140–144. Bibcode:1950PhRv...78..140G. doi:10.1103/PhysRev.78.140.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 0.5/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES, however you have written a lot of explanations in the "pre" boxes, it makes the text run off the screen so you can't read it.
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES, well done. Please note electron-negative is not a word, you mean electronegative.
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 2.5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES. You could have included more detail in your descriptions of the MOs however, such as which atom is contributing which AO to the MO, and whether the interactions are bonding or antibonding.
Independence 1/1
If you have finished everything else and have spare time in the lab you could:
Check one of your results against the literature, or
You checked a result against the literature, well done!
Do an extra calculation on another small molecule, or
Do some deeper analysis on your results so far