Blb15 2016-3-7
Getting started
NH3
File Name = BLB15_NH3_OPT_POP Calculation Method = RB3LYP Basis Set = 6-31G(d,p) E(RB3LYP) = -56.55776873 a.u. RMS Gradient Norm = 0.00000485 a.u. Imaginary Freq = 0 Point Group = C3V Bond Angle = 105.741 Bond Length = 1.01798
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000070 0.001800 YES RMS Displacement 0.000033 0.001200 YES Predicted change in Energy=-5.785205D-10
test molecule |
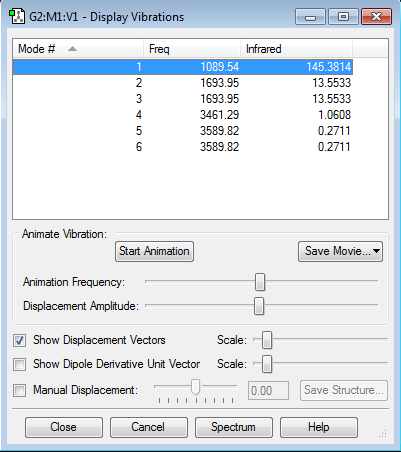
The number of six vibrational modes are expectable from the 3N-6 rule for nonlinear molecules (N is the number of atoms in the molecule). There are two pairs of degenerate modes, one pair of stretch at 1700 cm-1 and one of bending at 3600 cm-1. Additionally, the vibration at 1100 cm-1 is a bend (also known as umbrella), and the 3460 cm-1 is a stretch. Both of the not degenerate ones are very symmetric as they all have a C3 symmetry. Apparently only the lower two frequencies can be observed in IR spectrum due to the higher ones being really weak (13.5 and 145 vs 0.27 and 1.06) meaning that we would observe two peaks.
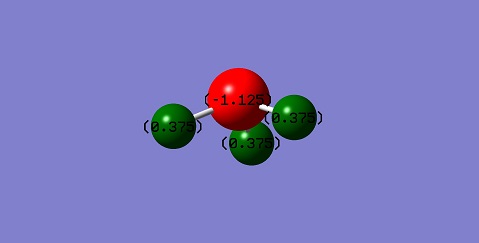
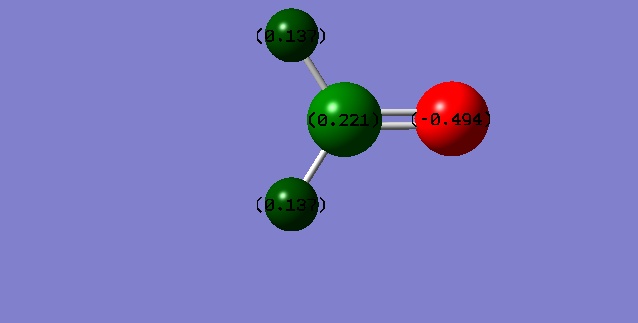
The charge distribution is according to expectations, the sum of the charges is zero, the hydrogen atoms are even and the nitrogen wears a relatively big negative charge, that can be expected from the difference in electronegativity values.
H2
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.164080D-13
File Name = BLB15 H2 POP File Type = .log Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = 6-31G(d,p) Charge = 0 Spin = Singlet E(RB3LYP) = -1.17853936 a.u. RMS Gradient Norm = 0.00000017 a.u. Imaginary Freq = 0 Dipole Moment = 0.0000 Debye Point Group = D*H Bond Length = 0.743
N2
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.401117D-13
File Name = BLB15 N2 POP File Type = .log Calculation Type = FREQ Calculation Method = RB3LYP Basis Set = 6-31G(d,p) Charge = 0 Spin = Singlet E(RB3LYP) = -109.52412868 a.u. RMS Gradient Norm = 0.00000060 a.u. Imaginary Freq = 0 Dipole Moment = 0.0000 Debye Point Group = D*H Bond Length = 1.11
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u.= -146.48 kJ/mol
This means that the ammonia is more stable thermodinamically, the reaction is exothermic. It does not quite fit the literature value of -92 kJ/mol, the error is due to the simulation process which would be different if we take the real circumstances into account, but that requires a deeper understanding of the software.
Simulation of formaldehyde
Basics
test molecule |
Vibrations
As a nonlinear molecule, the 3N-6 rule applies and can be observed:
 The modes visualised in order 1-6:
The modes visualised in order 1-6:
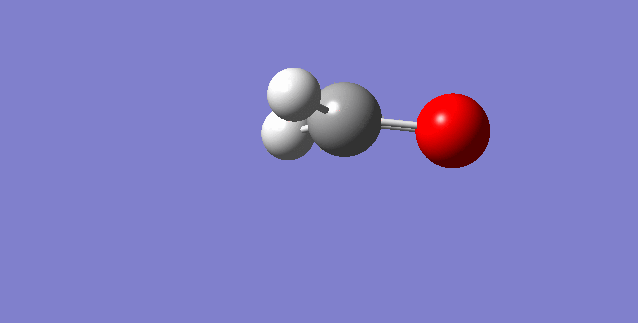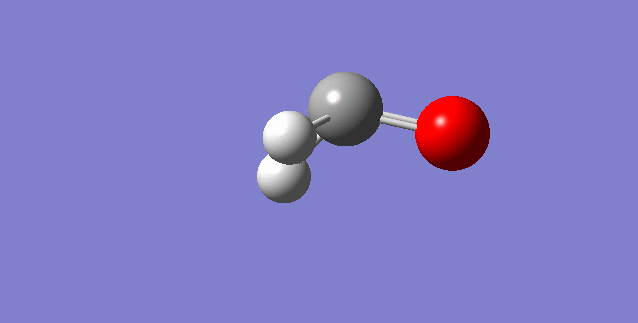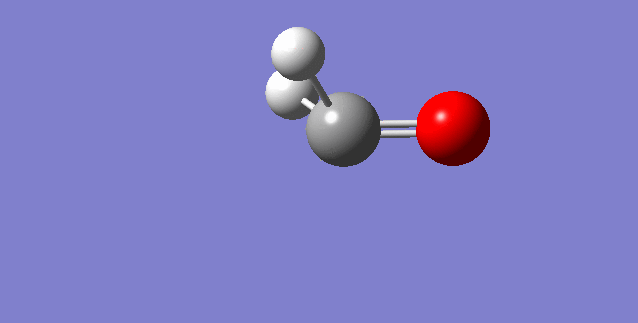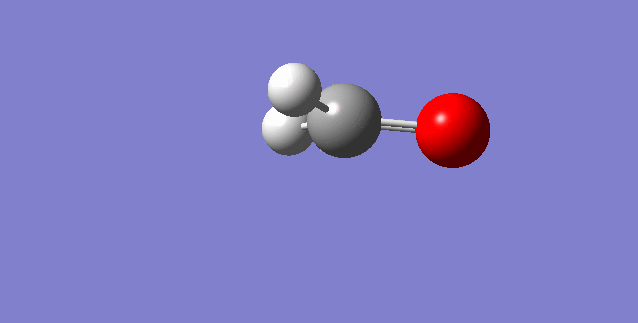
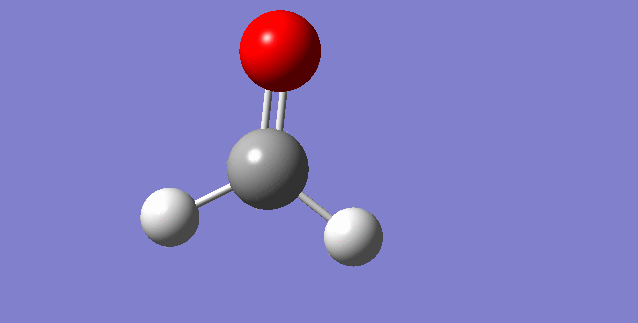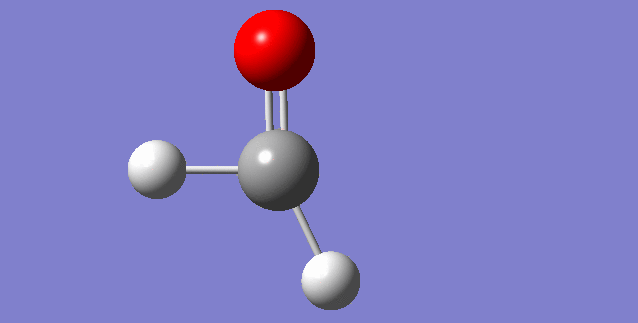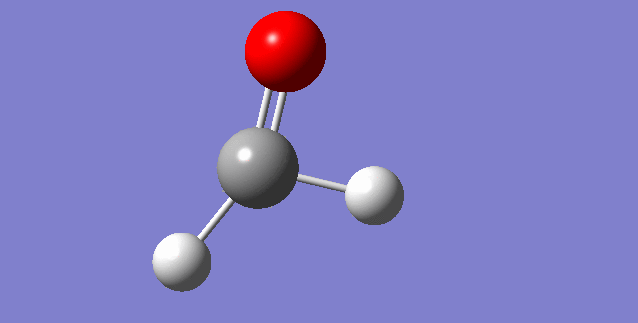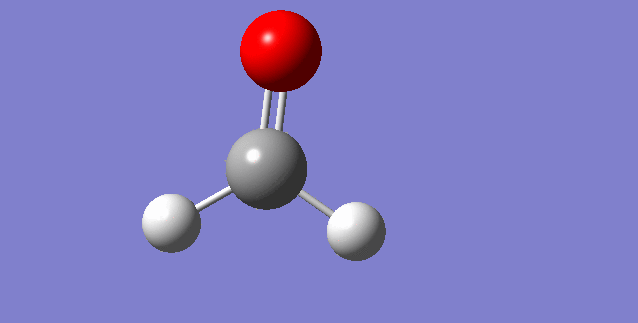
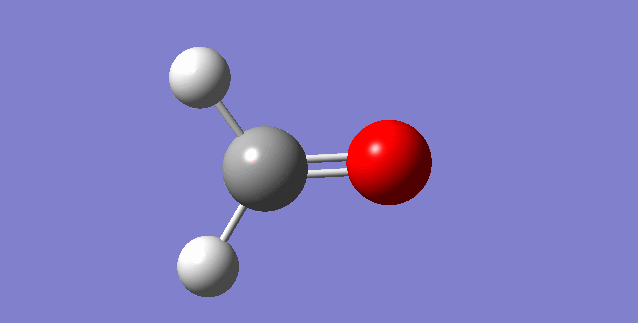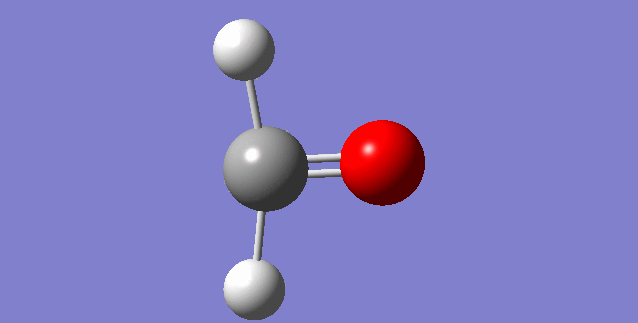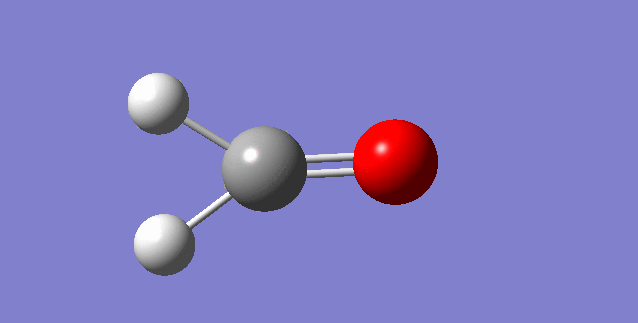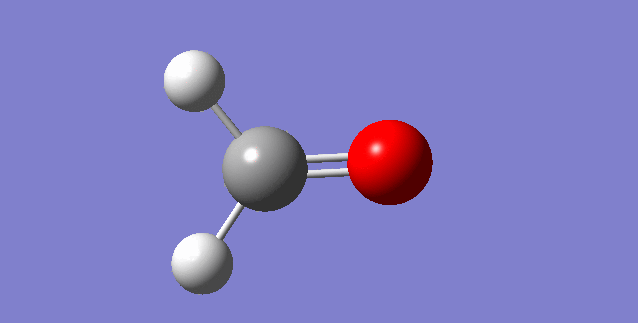
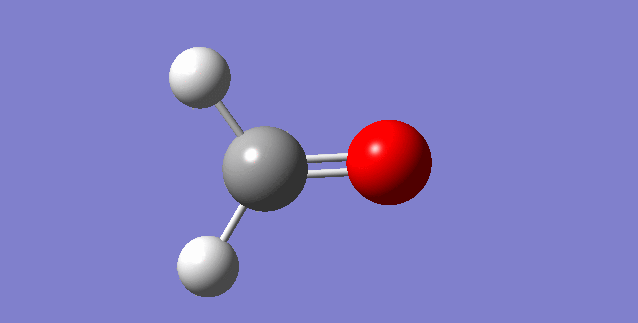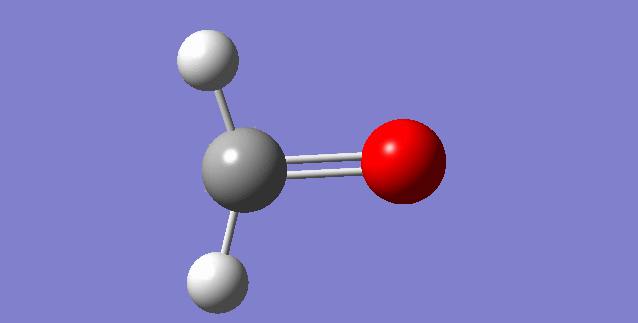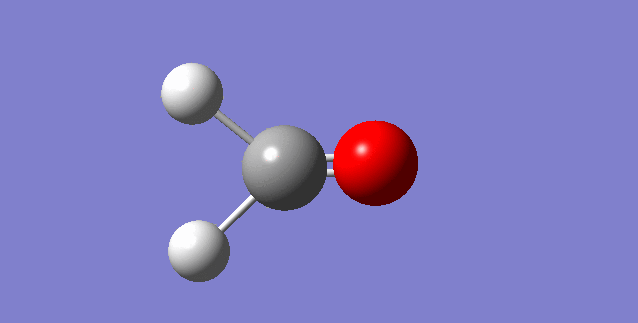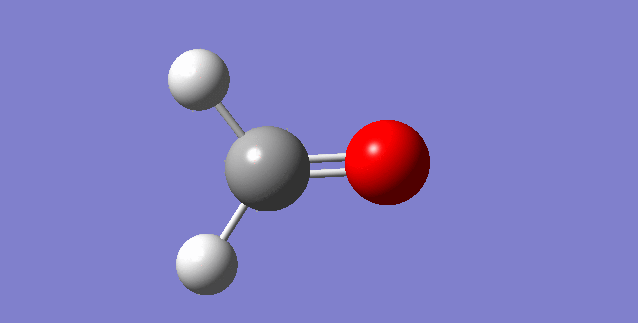
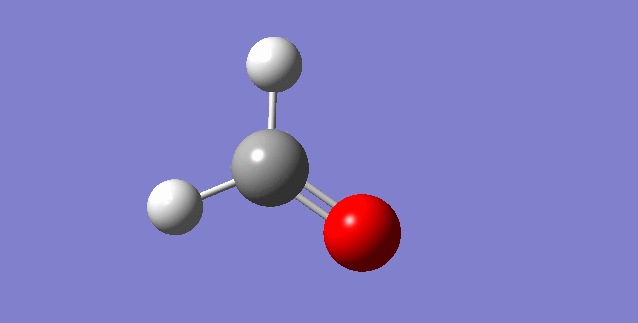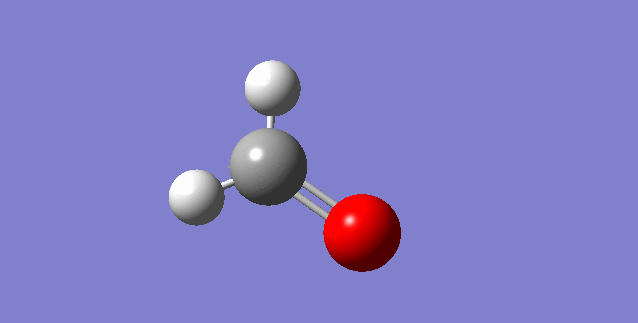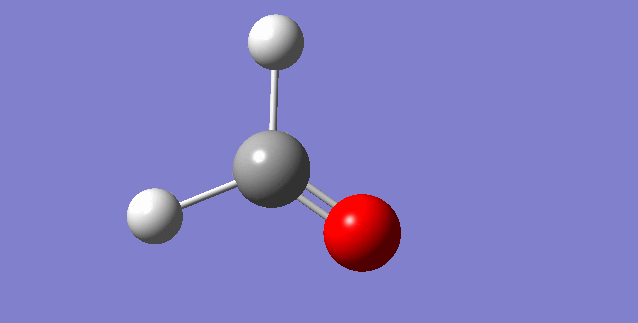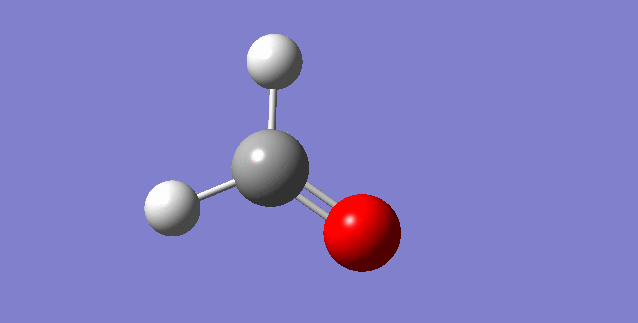
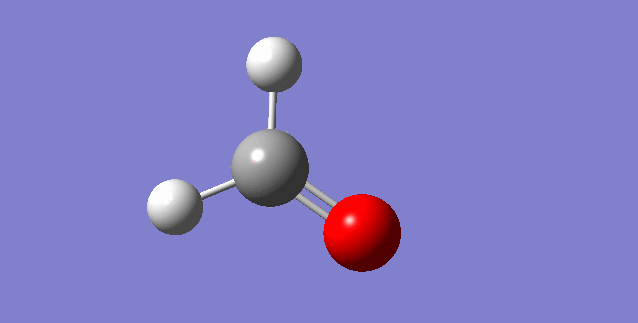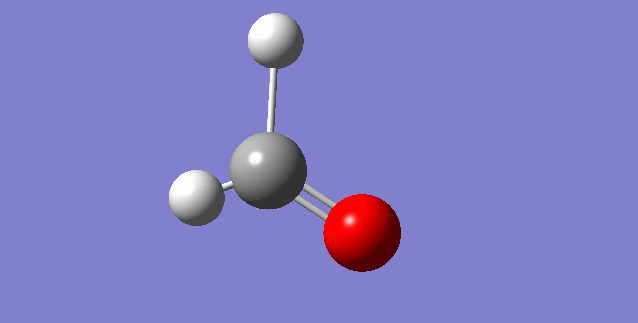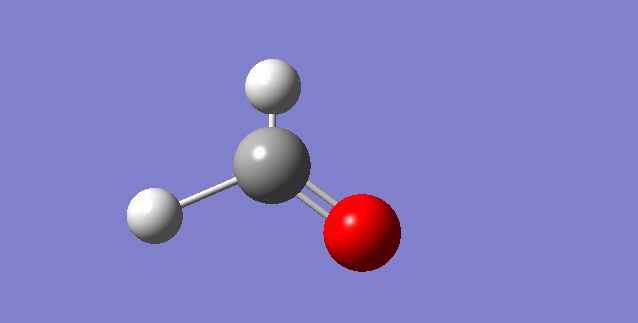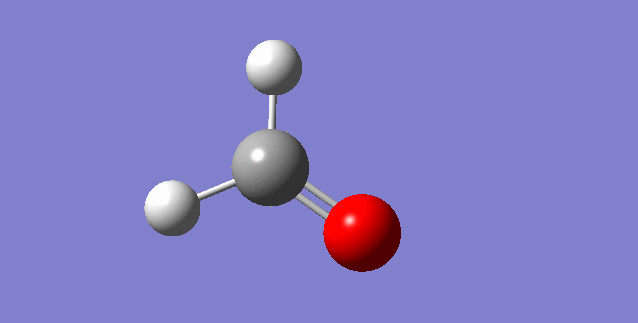
Charge distribution
The charges are according to expectations, the oxygen wears a positive partial charge, while the carbon and the hydrogens are positive, the carbon being the most positive.
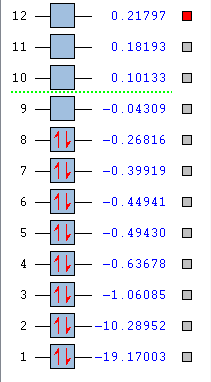
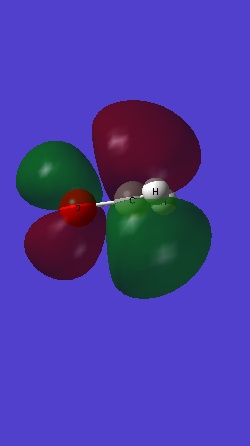
Molecular orbitals
MOs 1 and 2 involve the 1s electrons of oxygen and carbon in a nonbonding MO , localized tightly around C and O.
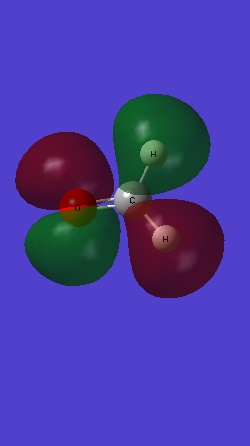
MO 3 is the σ bonding MO from 2s orbitals of C and O:
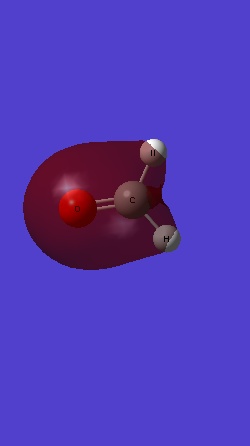
MO 4 is the σ* antibonding from MO 2s electrons of the two H:
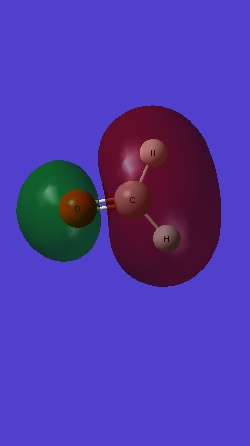
MO 5 is the is the bonding combination of 1s with the 2px of C and O:
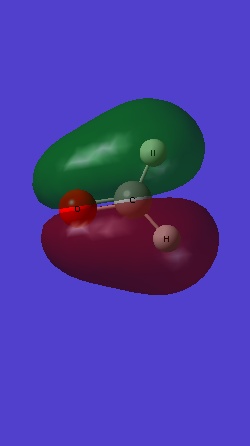
MO 6 is the bonding 2py of C and O:
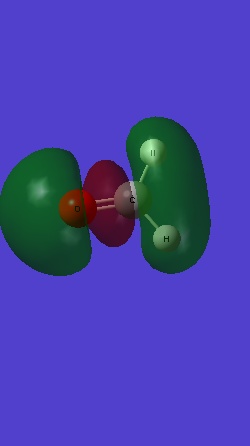
MO 7 is the bonding 2pz of C and O: