BD98
Inorganic Computational Lab Bence Dallos
Day 1 Exercises
BH3
B3LYP/3-21G level
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000053 0.001800 YES RMS Displacement 0.000027 0.001200 YES
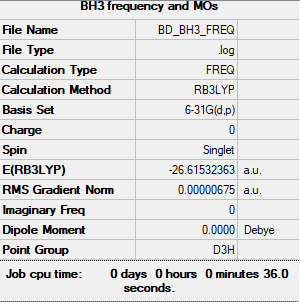
Frequency analysis file: BD_BH3_FREQ.LOG
Low frequencies --- -7.5936 -1.5614 -0.0055 0.6514 6.9319 7.1055 Low frequencies --- 1162.9677 1213.1634 1213.1661
optimised BH3 molecule |
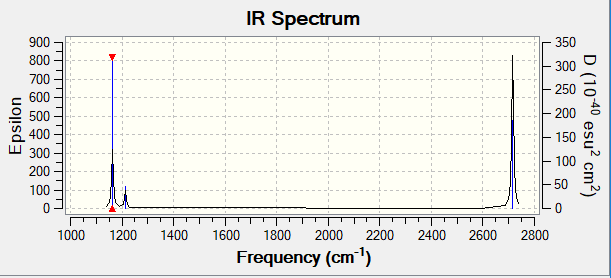
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A"2 | yes | Out-of-Plane bend |
| 1213 | 14 | E' | very slight | In-Plane Bend |
| 1213 | 14 | E' | very slight | In-Plane Bend |
| 2582 | 0 | A'1 | no | Symmetric Stretch |
| 2716 | 126 | E' | yes | Asymmetric Stretch |
| 2716 | 126 | E' | yes | Asymmetric Stretch |
There are only 3 peaks that appear in the IR spectrum. This is due to the fact that two vibrations that are degenerate appear(2716 cm-1) as one peak and there is one symmetric stretch which is not IR active and hence doesn't appear in the spectrum.
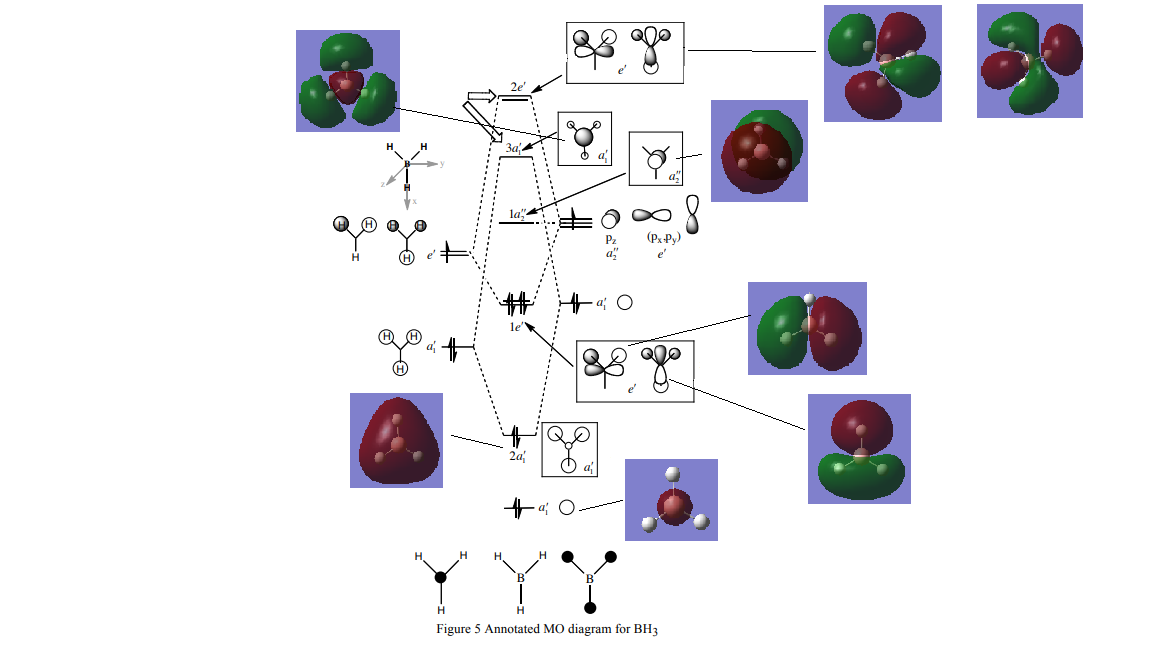
MO Diagram of BH3
Diagram taken from huntresearchgroup.org.uk : [1]
Are there any significant differences between the real and LCAO MOs? The lower energy MOs are very similar to the LCAO ones,however as you go up in energy they are increasingly harder to interpret. MOs up to the LUMO (plus 2/3 level) can be rationalised into LCAO MOs, upwards in energy from here, mathematical calculations are needed to evaluate them precisely.
Smf115 Good consideration of both the similarities and differences of the real and LCAO MOs. However, the trend identified is usually true because the MOs get more complex, although this isn’t always the case, and your reasoning could be clearer or specific differences (e.g. MO 3a1) could have been highlighted.
What does this say about the accuracy and usefulness of qualitative MO theory? Qualitative Mo theory is accurate to a certain degree, with simpler molecular orbitals.
NH3
B3LYP/3-21G level
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000014 0.001800 YES
RMS Displacement 0.000009 0.001200 YES
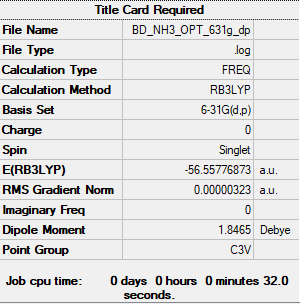
Frequency analysis file: BD_NH3_FREQ.LOG
Low frequencies --- -32.4235 -32.4224 -11.4276 -0.0047 0.0113 0.0476 Low frequencies --- 1088.7628 1694.0251 1694.0251
optimised ammonia molecule |
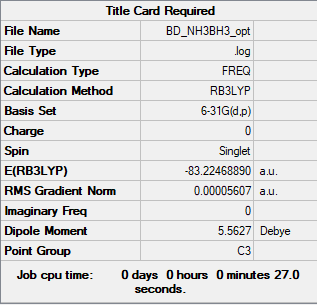
NH3BH3
B3LYP/3-21G level
Item Value Threshold Converged? Maximum Force 0.000164 0.000450 YES RMS Force 0.000035 0.000300 YES Maximum Displacement 0.001053 0.001800 YES RMS Displacement 0.000401 0.001200 YES
Frequency analysis file:BD_NH3BH3_FREQ.LOG
Low frequencies --- -0.0003 0.0010 0.0010 8.0372 12.6717 24.1905 Low frequencies --- 263.4702 631.3630 638.1909
Optimised NH3BH3 Molecule |
Association Energy Calculations
E(NH3)= -56.55777 a.u. = -1484492 kj/mol
E(BH3)= -26.61532 a.u.= -69879 kj/mol
E(NH3BH3)= -83.22469 a.u.= -218506 kj/mol
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]= -83.22469 a.u.- (-56.55777 a.u. + -26.61532 a.u.) =-0.0516 a.u.= -135 kj/mol
Ng611 (talk) 18:42, 8 May 2019 (BST) Good calculation, well done
The B-N dative bond is a weak dative bond. Dative bonds are slightly stronger than H-bonds and ionic bonds however weaker than regular covalent bonds due to their increased elasticity.
Ng611 (talk) 18:42, 8 May 2019 (BST) You need to compare your bond energies to those of other bonds from the literature.
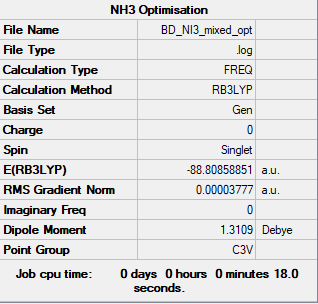
NI3
B3LYP/6-31G(d,p)LANL2DZ
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000044 0.000300 YES Maximum Displacement 0.000477 0.001800 YES RMS Displacement 0.000358 0.001200 YES
Frequency analysis file: BD_NI3_MIXED_FREQTEST.LOG
Low frequencies --- -12.7350 -12.7288 -6.2862 -0.0040 0.0188 0.0634 Low frequencies --- 101.0321 101.0328 147.4112
Optimised NI3 Molecule |
N-I bond distance: 2.18363 Å
Ionic Liquids: Designer Solvents Project
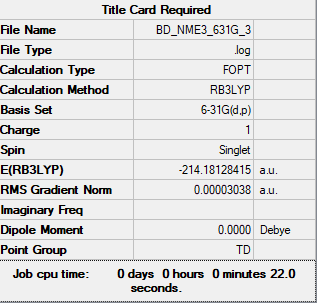
[N(CH3)4]+
B3LYP/3-21G level
Item Value Threshold Converged?
Maximum Force 0.000007 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000876 0.001800 YES
RMS Displacement 0.000223 0.001200 YES
Frequency analysis file: BD_NME3_FREQ8.LOG
Low frequencies --- -19.7959 -9.7560 -0.0006 -0.0004 0.0005 10.7730 Low frequencies --- 182.4925 281.7334 286.2752
Optimised [N(CH3)4]+ Molecule |
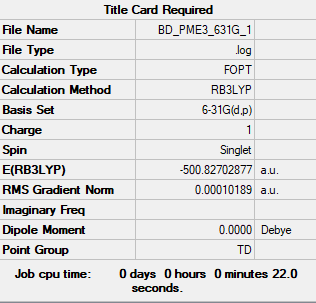
[P(CH3)4]+
B3LYP/3-21G level
Item Value Threshold Converged?
Maximum Force 0.000135 0.000450 YES
RMS Force 0.000075 0.000300 YES
Maximum Displacement 0.001611 0.001800 YES
RMS Displacement 0.000783 0.001200 YES
Frequency analysis file: BD_PME3_FREQ8.LOG
Low frequencies --- -24.5459 -0.0018 0.0011 0.0013 15.4554 23.7431 Low frequencies --- 154.3645 184.8166 191.9471
Optimised [P(CH3)4]+ Molecule |
Charge Analysis of Both Compounds
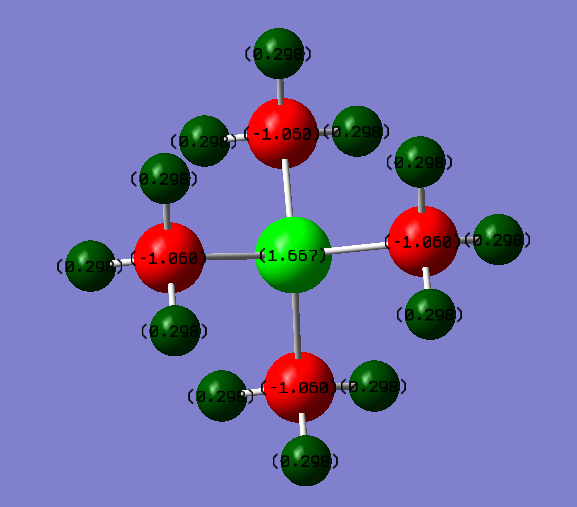
[P(CH3)4]+ :
| P | C | H |
|---|---|---|
| +1.67 | -1.06 | +0.30 |
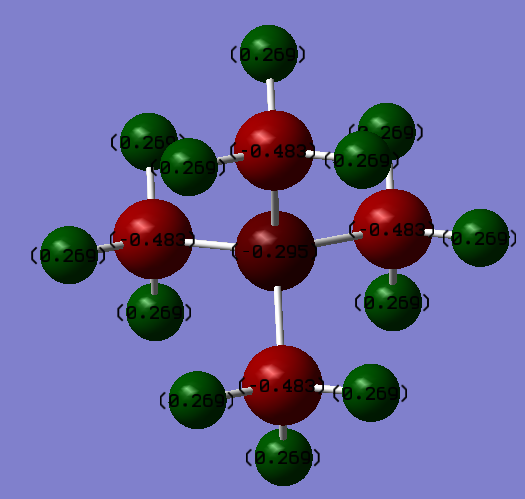
[N(CH3)4]+ :
| N | C | H |
|---|---|---|
| -0.30 | -0.44 | +0.27 |
For [P(CH3)4]+ Phosphorous is more electropositive than C, hence has a more positive charge.
In the case of [N(CH3)4]+ ,Nitrogen is more electronegative than Carbon hence has a negative charge.
For both molecules, the Hydrogen charges are the very similar, as the C-H electronegativity difference is not significantly affected by differing electronegativities on the Carbon.
Ng611 (talk) 18:49, 8 May 2019 (BST)What about symmetry effects?
Formal Charge Questions
What does the "formal" positive charge on the N represent in the traditional picture? The formal charge is the overall charge assigned to the nitrogen atom, assuming that all the electrons in the chemical bonds are shared equally and disregarding electronegativity. In the charge distribution, the electronegativities are taken into account hence the charge is on nitrogen is no longer +1.
On what atoms is the positive charge actually located for this cation?The actual charge is located on the Hydrogen atoms, as seen in the charge distribution picture.
Molecular Orbital Analysis of [N(CH3)4]+
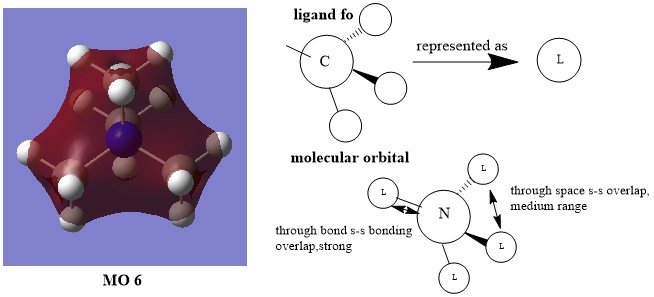
MO6:
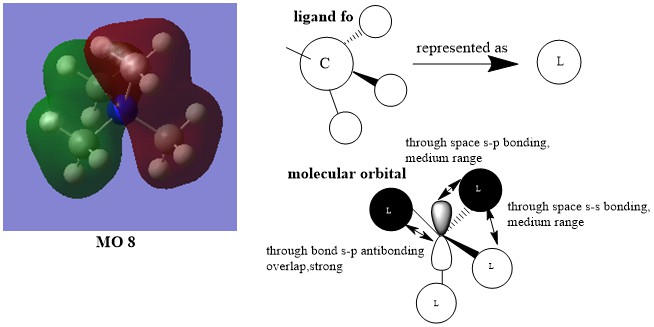
MO8:
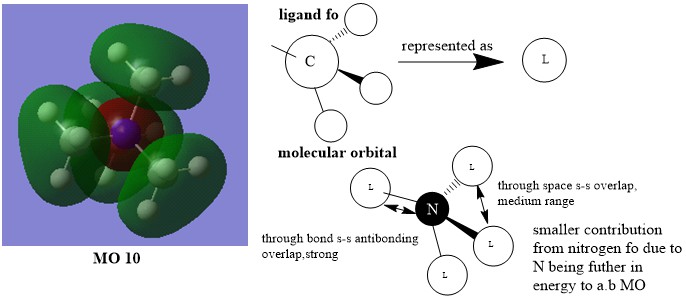
MO10:
Smf115 The FOs and the LCAOs have been correctly assigned and a good attempt at highlighting some of the interactions. To improve, the drawing of the LCAO/FOs could be consistent across the three and a greater range of MO complexity/character could have been chosen.
Smf115 Overall, a very good report which is consistent across both sections.