Aspartame
| Aspartame | |
|---|---|
| |
| General | |
| Systematic name | N-(L-α-Aspartyl)-L-phenylalanine
methyl ester |
| Other names | 3-amino-N-(1-methoxycarbonyl-
2-phenyl-ethyl)-succinamic acid |
| Molecular formula | C14H18N2O5 |
| SMILES | COC([C@H](CC1=CC=CC=C1)NC
([C@H](CC([O-])=O)[NH3+])=O)=O |
| Molar mass | 294.30gmol-1 |
| E number | E951 |
| Appearance | White Crystal or Crystalline Powder |
| CAS number | 22839-47-0 |
| Melting point | 519-520 K |
| Boiling point | Decomposes |
| Chiral rotation [α]D | +14.5° to +16.5°[1] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Aspartame

XML error: Mismatched tag at line 16
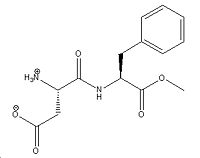
Aspartame is an artificial non-saccharide sweetener used in thousands of foods and beverages worldwide. Aspartame is made by essentially joining L-phenylalanine, L-aspartic acid and a methyl ester group.[2]
Even though it is over 180-200 times sweeter than suger and virtually non-caloric, it is a controversial compound because of its possible health risks, which only came to the public's attention once studies began in 1995, reporting of its adverse effects, 24 years after it was first used.
Synthesis
There are many different ways to synthesise aspartame. For example:
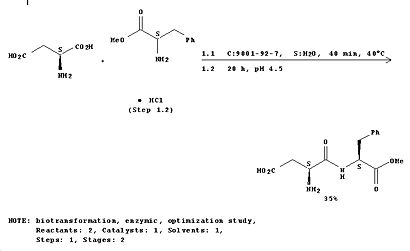
1. The following reaction consists of L-aspartic acid reacting with L-phenylalanine methyl ester in the presence of a thermostable protease enzyme in the temperature range of 20-75°C and pH range of 4.5-7.8 for 2 to 20 hours. After removing any side products and excess, the aspartame is obtained.
2. Preparing a mixture of molten N-benzyloxycarbonyl-L-aspartic acid and 20M L-phenylalanine methyl ester mixtures at 50°C.[3]DOI:10.1080/1024242021000058676
Health Risks
The FDA(Food and Drug Administration), who are responsible for the public of USA, has set the acceptable daily intake for aspartame at 50 mg/kg of body weight per day. Aspartame is digested when we ingest it, breaking down into phenylalanine, aspartate, and methanol. When the liver breaks down methanol, it is released as formic acid and formaldehyde.
Formaldehyde
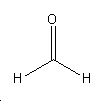 Formaldehyde is known to have health risks[4]:
Formaldehyde is known to have health risks[4]:
- It is toxic if swallowed.
- Interferes with DNA replication.
- Causes birth defects.
- Carcinogenic effect, however only if it is the body for long periods of time.
Formic acid
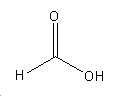 Formic acid is known to have a health risks[5]:
Formic acid is known to have a health risks[5]:
- It prevents a cell's mitachondria from producing ATP, hence all cellular processes stop due to a lack of energy.
- Optic nerve cells are very sensitive to formic acid, which is why methanol poisoning is associated with blindness.
Reaction
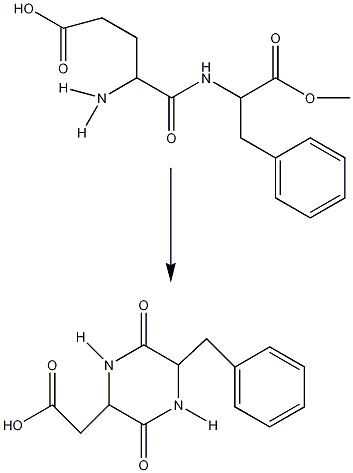
Intermolecular conversion of Aspartame to 3-(carboxymethyl)-6-benzyl-2,5-diketopiperazine(DKP)
Aspartame will undergo a cyclization reaction to DKP when heated:
This is important to look at because the sweetener will be used at high temperatures e.g. in hot drinks. The production of DKP and loss of Aspartame can be measured using FT-IR spectroscopy to look for the loss and gain of characteristic functional groups.
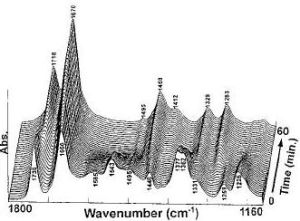
Looking at the IR it can be seen that when the reaction has not started the IR is that of Aspartame. As the reaction goes on the disappearance of the N-H peak (1543cm-1) and the C-O ester peak(1225cm-1)as well as the exchange of the carbonyl peak(ester)(1736cm-1) for a carbonyl peak(acid)(1718cm-1) shows the conversion between these two molecules. This reaction however only happens above 153°C which means there is no danger of reaction at the water boiling temperature of 100°C.
Spectroscopy/Spectrometry
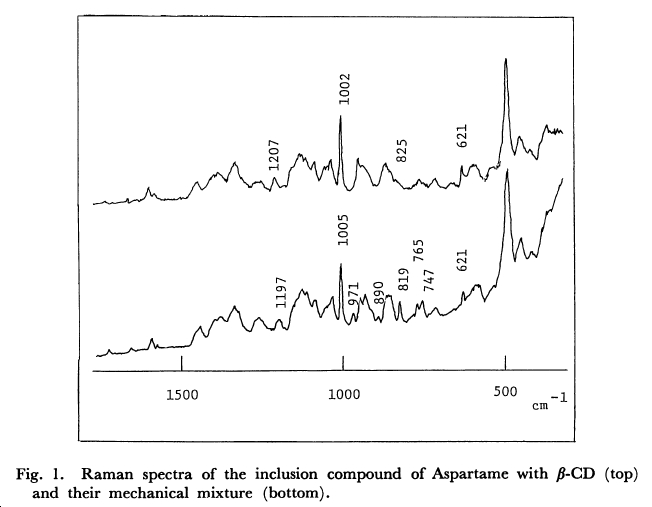
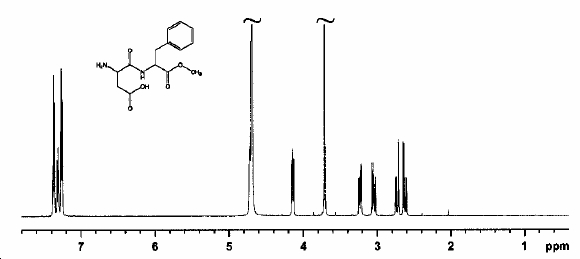
Raman Spectrum of Aspartame with β-Cyclodextrin
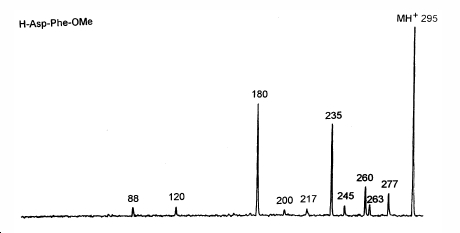
Mass Spectrum of Aspartame
NMR Spectrum of Aspartame
References
- ↑ http://www.spectrumchemical.com/retail/product.asp?catalog%5Fname=Chemicals&category%5Fname=&product%5Fid=5438147#
- ↑ http://www.aspartame.org/aspartame_factsheet.html
- ↑ http://www.informaworld.com/smpp/content?content=10.1080/1024242021000058676
- ↑ http://www.sigmaaldrich.com/catalog/search/ProductDetail/SIGMA/F8775
- ↑ http://recipes.howstuffworks.com/question536.htm
- ↑ AU: Alex. G. Harrison, Ya-Ping Tu TI: Ion chemistry of protonated aspartic acid derivatives SO: Journal of Mass Spectrometry VL: 33 NO: 6 PG: 532-542 YR: 1998 CP: Copyright © 1998 John Wiley & Sons, Ltd. ON: 1096-9888 PN: 1076-5174 AD: Department of Chemistry, University of Toronto, Toronto, Ontario M5S 3H6, Canada
- ↑ Structure, Dynamics, and Stability of β-Cyclodextrin Inclusion Complexes of Aspartame and Neotame Garbow, J.R., Likos, J.J., and Schroeder, S.A. J. Agric. Food Chem., 49, 4, 2053 - 2060, 2001