Ajm3081
Introduction
Computer modelling is becoming an ever more powerful and important tool in predicting the outcome of chemical reactions, including regioselectivity, stereoselectivity as well as the relative stability of major and minor products. The aim of this project is to gain a basic understanding of the techniques and applications of a range of computational methods
Hydrogenation of the cyclopentadiene dimer
Cyclopentadiene dimerisation
Cyclopentadiene reacts in a [4+2] cycloaddition reaction to yield as the major product the endo form. The selection of the endo form can either be attributed to thermodynamic or kinetic control.
Chem3D was used to model both the endo and the exo form and the MM2 force field was used for geometry optimisation. Total relative energies for the two possible products are shown below:
Exo Product: 31.88 kcal/mol Endo Product: 34.01 kcal/mol
It can be deduced from looking at the above figures, that the reaction must indeed be under kinetic control. The endo product is less thermodynamically stable than the exo product so the reaction cannot be under thermodynamic control.
The kinetic control shown in this reaction, can be attributed to the more favourable orbital overlap situation in the endo configuration. This is shown in the diagram below:
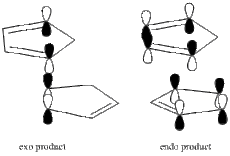
Hydrogenation of the cyclopentadiene dimer
The favoured endo product in the initial dimerisation was then to have hydrogenation modelled. With two available double bonds which could undergo hydrogenation, there are again, two different products. The major product yielding from this reaction will either be under kinetic or thermodynamic control. The two possible products were modelled and then had geometry optimisation performed, again using the MM2 force field.
| Product 1 | Product 2 | |
|---|---|---|
| Stretching | 1.28 | 1.09 |
| Bending | 19.80 | 14.52 |
| Torsion | 10.87 | 12.50 |
| Van de Waals | 5.64 | 4.51 |
| Dipole/dipole | 0.16 | 0.14 |
| Energy (kcal/mol) | 35.70 | 31.15 |
The table shows valuable information obtained from the geometry optimisation calculation, showing the contributions to the total energy of the molecule, made from a number of other modes of energy. Product 2 has a lower total energy than Product 1, by 4.54 kcal/mol and is therefore the thermodynamic product of this reaction.
The largest contribution to the difference in energies between the two possible products comes from the torsional strain and the bending terms. Product 1 has higher values for both of these modes.
In product 2, the torsional strain is greater than in product 1. This indicates that it is preferable for the cyclopentadiene dimer to be hydrogenated as in the case of product 1 with respect to torsional strain. However, the higher bending contribution in product 3 outweighs the decrease in torsional strain and as such product 2 is preferred.
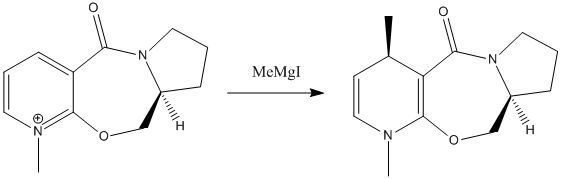
Stereochemistry of nucleophillic addition to pyridinium ring (NAD+ analogues)
Reaction 1
Once again, the initial step was to model the reactant and then use the MM2 force field method to perform geometry optimisation. A range of possible conformers were modelled and calculations were performed upon each. As expected, each conformer had different geometric characteristics and different thermochemical characteristics.
Dihedral angles were measured around the carbonyl functional group.
5 conformers were modelled, and created by repositioning of both the 5-membered ring and the ethereal oxygen. Due to the rigid nature of the aromatic portion and the carbonyl groups, no changes were made to this section of the molecule. Repositioning above, below and in plane with the aromatic portion led to 5 conformers. Results from the geometry optimisation are shown below:
| Property | Conformer 1 | Conformer 2 | Conformer 3 | Conformer 4 | Conformer 5 |
|---|---|---|---|---|---|
| 5-membered Ring | above plane | above plane | below plane | below plane | flat |
| Ethereal Oxygen | above plane | below plane | above plane | below plane | flat |
| Energy of Molecule (kcal/mol) | 44.41 | 44.62 | 44.70 | 43.11 | 43.13 |
| Dihedral Angle | 23.8 | 12.2 | 23.7 | 10.9 | 9.0 |
The most stable conformer is the case where both the ethereal oxygen and the 5 membered ring are positioned below the planar aromatic portion of the molecule.
An optimum dihedral angle of 10.9 degrees was calculated, but the results also show that the carbonyl functional group is always located on the top face of the molecule. This constant location of the carbonyl group across all conformers gives rise the selective nature of methyl addition to the top face of the molecule. The grignard reagent used is able to coordinate the carbonyl oxygen on the top face of the molecule, and as such addition of the methyl group must occur on to the top face.
Limitations of the methodology used include the inability to factor in the grignard reagent when carrying out the calculations. This would be sure to make the calculations more representative of reality.
Reaction of pyridinium ring with aniline

The above scheme shows the reaction of aniline with pyridinium ring. Stereoselectivity is once again present in respect to the position of addition of the pyridinium ring.
In order to find the origin of this control, the reactant in the reaction was defined and the MM2 force field was used to optimise the geometry. Different conformers of the reactant were drawn and minimised using the MM2 force field, with the focus lying on the geometry of the carbonyl group. This gave different minimized geometries with different total energies and dihedral angles. Dihedral angles were measured using the carbonyl carbon and oxygen, along with the adjacent aromatic carbon, and the aromatic carbon adjacent to that one.
Once again, the reactant was modelled and the MM2 force field used to optimise geometry. Again, different possible conformers were modelled and optimised. Dihedral angles and total energy were measured.
The conformers were produced by repositioning of both the carbonyl group and the tertiary nitrogen group either above or below the plane of the molecule. Different permutations of these positions were modelled and data for each conformer are shown below:
| Property | Conformer 1 | Conformer 2 | Conformer 3 |
|---|---|---|---|
| Carbonyl Group | Above plane | Above plane | Below plane |
| Tertiary Nitrogen Group | Above plane | Below plane | Below plane |
| Energy (kcal/mol) | 84.17 | 63.74 | 63.55 |
| Dihedral Angle | 22.6 | -16.8 | -18.1 |
The lowest energy conformation has a dihedral angle of -18.1 degrees and has both the carbonyl group and the tertiary nitrogen group below the plane of the molecule.
Once again, the top face of the molecule is the site for addition. Attack occurs to the opposite face of the molecule from the carbonyl group in order to avoid any steric issues. As the most stable conformer has the carbonyl group on the bottom face of the molecule, the aniline is compelled to add to the top face.
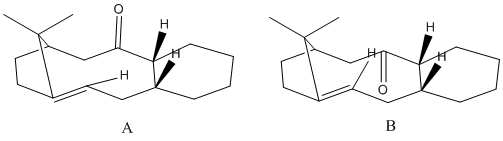
Stereochemistry and reactivity of an intermediate in the synthesis of taxol
Two isomers of an important intermediate in the production of Taxol are shown below:
The type of isomerism present is atropisomerism. This occurs as a result of the impedence of rotation around a single covalent bond in a molecule. This impedence gives rise to stereoisomers.
Isomer A has the carbonyl group upwards, whereas isomer B has the carbonyl group downwards. The two isomers were modelled and geometries were optimised using the MM2 force field. The MMFF94 force field was also utilised in geometry optimisation. The table below shows the results:
| Molecule | A | B |
|---|---|---|
| MM2 Energy (kcal/mol) | 55.32 | 49.43 |
| Torsion (kcal/mol) | 20.17 | 17.51 |
| MMFF94 Energy (kcal/mol) | 77.60 | 70.66 |
Isomer B has a lower total energy than isomer A. Both of the methods used - force fields MM2 and MMFF94 - reach the same conclusion although the absolute energy levels are different. The energy difference between the two isomers is very similar in both cases.
Modelling using semi-empirical MO theory: Regioselective addition of dichlorocarbene
9-ChIoro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene was modelled in ChemBio3D and then the geometry was optimised using the MM2 force field to yield a total energy of 17.90 kcal/mol.
The MOPAC/RM1 method was then utilised in order to produce an approximation of the valence electron molecular orbitals.
 |
 |
 |
 |
 |
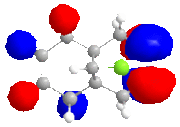 |
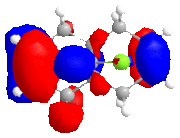
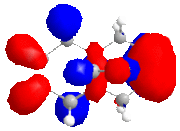
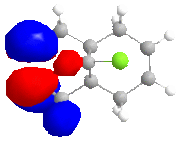
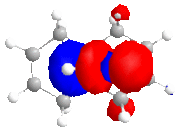
The calculated approximate molecular orbitals shown above can give an insight in to the control that orbitals are able to have on reactivity. In this case we will be looking at the cycloaddition of dichlorocarbene to the alkene double bond in the starting material.
The approximate molecular orbitals show that in the HOMO of the molecule, there is greater electron density in the alkene double bond endo to the chlorine atom. As this double bond has more electron density in the HOMO than in the bond exo to the chlorine atom, it will be more liable to electrophillic attack than the other double bond.
The intramolecular distances between the exo and endo double bond carbons and the central bridgehead carbon was measured on the geometry optimised model.
| Exo carbon to central bridgehead carbon | Endo carbon to central bridgehead carbon | |
|---|---|---|
| Distance (Angstrom) | 2.98 | 3.22 |
The molecule is clearly distorted, with bending of the exo double bond towards the bridgehead carbon to a greater extent than the endo double bond. There is present, an antiperiplanar relationship between the exo pi orbital and the Cl-C sigma* orbital. The interaction would lead to stabilisation of the exo double bond, thus making it less susceptible to eletrophillic attack.
The product from the hydrogenation of the exo double bond was then modelled and was geometrically optimised using the MM2 force field to give an energy of 24.82 kcal/mol.
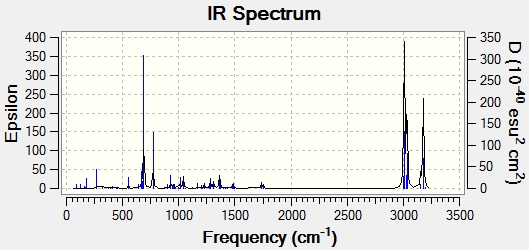
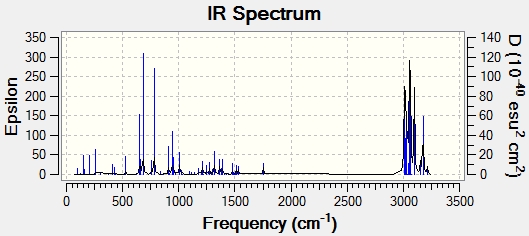
Both optimised structures were then subjected to a Gaussian calculation in order to calculate the vibrational stretching frequencies and IR spectra:
 |
 |
| 9-ChIoro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene | Hydrogenated product | |
|---|---|---|
| C=C bond stretch frequency (cm-1) | 1757.4 | 1753.7 |
| C=C bond stretch frequency (cm-1) | 1737.1 | - |
| C-Cl bond stretch frequency (cm-1) | 770.9 | 780.4 |
C-Cl stretching occurs at different frequencies in each of the molecules, with the diene having a lower stretching frequency implying that the bond is weaker. Overlap between C-Cl sigma* and the exo pi orbitals would serve to increase electron density in the C-Cl antibonding orbital thus making it weaker. The exo double bond is not present in the dihydo derivative so no weakening of the C-Cl bond occurs and the bond is stronger, so has a higher stretching frequency.
C=C stretching at 1757 /cm can be attributed to the endo double bond, and is therefore present in both the diene and the dihydro derivative. There is of course an additional C=C stretch in the diene. The frequency of the stretch is lower, indicating a weaker, longer bond. The calculated molecular orbitals would seem to concur with these results. The lower electron density in the exo C=C bond would lead to a weaker bond with lower stretching frequency.
Structure Based Mini Project
Regio- and Stereo-selective conversion of Alkenes to Epoxides
I will be investigating the stereo- and regio-selective conversion of alkenes to epoxides. The epoxidation of the 1,3-diene shown below yields either one of two products, also shown below. Molecular modelling will be used to obtain a variety of spectroscopic data for each potential epoxide product, which will then be compared to experimental date from the literature. Confirmation of the expected product is therefore possible using molecular modelling.
The epoxide formed during the epoxidation of the 1,3-diene is dependent upon the type of reagents used in the epoxidation. Epoxidation will usually take place stereoselectively on the least hindered face of the diene. If a bulky reagent is used,such as mCPBA, then the steric clash between the reagent and the dioxolane group leads to the reaction being forced on to the opposite face of the molecule. The epoxide and the dioxolane group are located on opposite faces of the molecule.
Direction of the reaction is possible, and cases where epoxidation on to the most hindered face have been reported in the literature. In the synthesis of the species with epoxide and dioxolane on the same face of the molecule, a hydroxyl group is added to the aromatic portion of the molecule. Hydrogen bonding is able to direct the epoxidation to the same face as the dioxolane group.
Comparison of NMR Data provided by Guassian Calculation with that of Literature
Calculated NMR Spectral data [13C & 1H] is included below. Experimental data is also shown.
13C NMR
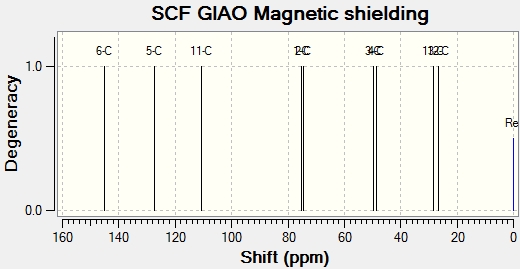
13C Calculated NMR Data [ppm]: 26.8 (s), 28.3 (s), 48.6 (s), 49.8 (s), 74.3 (s), 75.0 (s), 110.6 (s), 127.4 (s), 144.9 (s).
13C Experimental NMR Data [ppm][1]: 25.2 (s), 27.1 (s), 50.2 (s), 54.5 (s), 73.9 (s), 76.8 (s), 108.7 (s), 125.5 (s), 130.0 (s).
The experimental data collected from literature for the 13C NMR is consistent with that produced by the gaussian calculation. The chemical shifts are very similar in each case and demostrate that this particular molecular mechanics method is a reliable way of predicting the NMR of an expected product.
Experimental NMR data is consistend with calculated NMR data indicating that the methodology used is an accurate and reliable way of predicting NMR shifts.
1H NMR
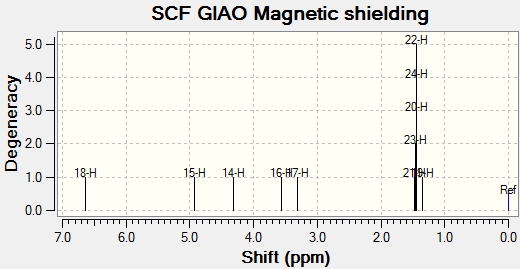
Calculated 1H NMR Data [ppm]: 1.4 (s, 3H), 1.5 (s, 3H), 3.3 (dd, J = 5.0, 5.0 Hz), 3.6 (m, 1H), 4.3 (dd, J = 6.1, 2.8 Hz, 1H), 4.9 (dd, J = 6.7, 1.9 Hz), 6.6 (d, J = 5.0 Hz, 1H).
Experimental 1H NMR Data [2]: 1.4 (s, 3H), 1.5 (s, 3H), 3.4 (dd, J = 4.5, 4.5 Hz, lH), 3.6 (m, lH), 4.5 (dd, J = 6.6, 2.4 Hz, lH), 4.7 (dd, J = 6.6, 1.8 Hz, lH), 6.7 (d, J = 4.5 Hz, 1H)
Again molecular modelling methodology has proved accurate in predicting shifts for 1H NMR. Using Jannochio, accurate 3J-J couplings values have also been found.
IR Spectrum
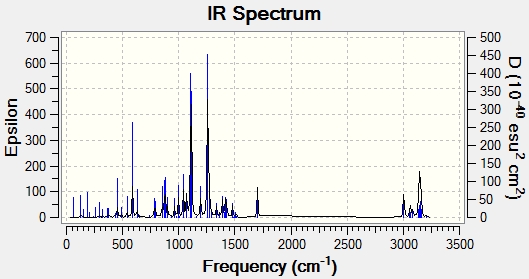
| IR Frequency/cm^-1 | Stretch/Bend |
| 3094 | C-H Stretch Aromatic |
| 2856 | C-H Alkane |
| 1603 | C=C Stretch Aromatic |
| 1267 | C-0 |
| 588 | C-Br |
UV/Vis Spectrum
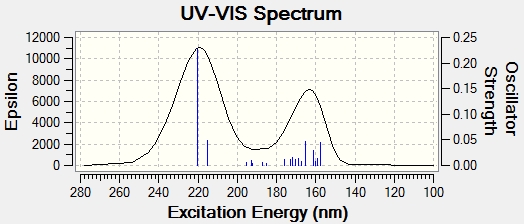
Max wavelength = 220.32nm
Optical Rotation
Optical rotation is the only possible way of deducing which enantiomer is present. Calculated optical rotations are -80.1 for the molecule with both groups on the same face, and +83.4 for the molecule with groups on opposite sides. This is expected as enantiomers by definition rotate plane polarised light in opposite directions to an equal extent.
Conclusions
It is clear that in the above work, computational methods can be relied upon to accurately predict the reality of chemistry. I have no doubt that as computing power becomes more cost effective and efficient, it will be relied upon heavily as a tool in synthesis, and will make a real impact in the more conventional sides of synthetic and practical chemistry.