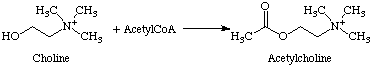Acetylcholine
Acetylcholine
| Acetylcholine | |
|---|---|
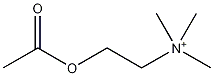
| |
| General | |
| Systematic name | 2-acetoxy-N,N,N-trimethylethanaminium |
| Other names | ACh |
| Molecular formula | C7H16NO2 |
| SMILES | CC(OCC[N+](C)(C)C)=O |
| Molar mass | 147.22gmol-1 |
| CAS number | 51-84-3 |
Acetylcholine is a neurotransmitter and was the first of its kind to be discovered, by Henry Hallett Dale in 1914. It is an ester of acetic acid and choline and is the neurotransmitter released upon stimulation of the vagus nerves.
3D Structure
Synthesis
Acetylcholine is synthesised by the reaction of choline with acetyl coenzyme A (acetyl CoA) and is catalysed by choline acetyltransferase, a biological enzyme.
Once the acetylcholine has performed its function, it is degraded by acetylcholinesterase, another important biological enzyme.
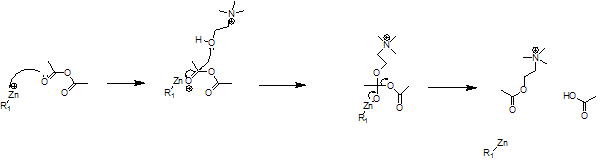
Mechanism
The R1 group refers to the remainder of the acetylcoenzyme A molecule. It actually has 4 bonds to the zinc atom, but these are not shown for simplicity. The reaction is a simple nucleophillic substitution on a carbonyl group. The ethanoic acid that is formed as a side product is broken down for respiration.[2]
Function
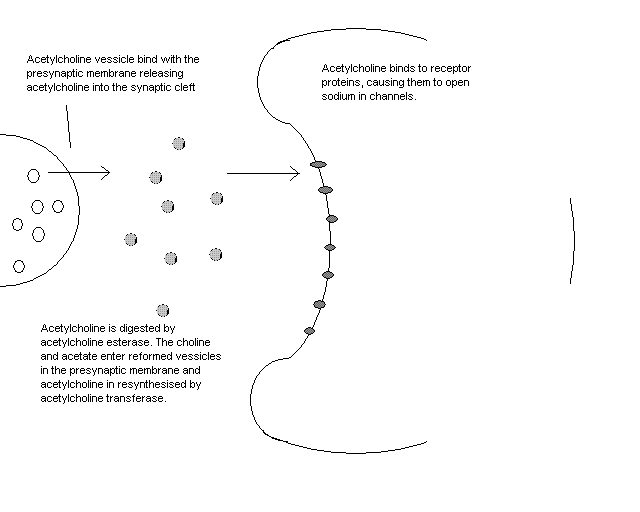
Acetylcholine has uses in both the central nervous system (CNS), and the peripheral nervous system (PNS). In the peripheral nervous system (ie nervous pathways not involving the brain and spinal chord) acetylcholine is one of the main neurotransmitters involved in the autonomic nervous system. The autonomic nervous system is involved in regulation and homeostasis. In the autonomic nervous system acetylcholine stimulates muscles by activating receptors on the cell membranes of target cells, which then release sodium ions into the intended cell, which in turn triggers an action potential, resulting in muscle contraction.
Function Mechanism
Acetylcholine is present in the synaptic knob of each autonomic relay neurone. It is released in the presence of a positive ionic potential. This is caused by the flow of sodium ions into the cell.
Acetylcholine esterase is the enzyme used to digest acetylcholine. This enzyme is present in the gap between neurones at synapses in the autonomic nervous system. This means that a large number of molecules of acetylcholine must be made for some to reach the receptors on the post-synaptic membrane. The receptors are transport proteins that are activated by the acetylcholine to allow sodium ions to enter the neurone. The acetylcholine receptors have multiple binding sites for acetylcholine, but a relatively low affinity for the molecule. However, each acetylcholine molecule that binds to the receptor protein opens the adjacent binding sites, making it easier for further acetylcholine molecules to bind. Thus, the presence of enough acetylcholine will greatly distort the protein, opening the channel to allow sodium ions to cross the post-synaptic membrane and continue the nervous impulse.
The acetylcholine is recycled, when the reactant materials re-enter the synaptic knob in vesicles. This is done using acetylcholine transferase.
Nicotine and carbamylcholine can also bind to the nicotinic acetylcholine receptors. This implies that acetylcholine binds to the receptors via the N-CH3 group on the choline.
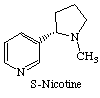
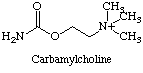
The post-synaptic membrane (like all organic membranes) is adaptable. It is rebuilt differently each time, depending on the environment. Hence, when nicotine is used, less nicotinic acetylcholine receptors are made present in the post-synaptic membrane. This makes it less receptive to acetylcholine as well as nicotine, making the person need more stimulation to gain the same mood effects. This makes nicotine and other drugs that interfere with the function of acetylcholine very addictive. This is combined with a lower quantity of acetylcholine being produced. Alternatively, some drugs inhibit acetylcholine esterase. This means that the acetylcholine continues to stimulate the reciever neurone after the initial nervous impulse. At small doses this increases brain activity by effectively increasing acetylcholine levels. It can also improve memory as shown by various trials on rats and monkeys. [3]
Uses
Acetylcholine has been used directly as a treatment for certain conditions. Its action in the body can be altered, by either blocking its effects altogether, or by merely hindering its effects. It can also be introduced to the body to certain target areas. For example it can be used during eye surgery to induce rapid pupil constriction. Also, inhibitors which stop acetylcholinesterase degrading acetylcholine are used to counter the effects of muscle relaxants, and are also found in certain medications used to treat Alzheimer’s.
References
- http://www.neurosci.pharm.utoledo.edu/MBC3320/acetylcholine.htm
- C. Y, Hong, L. J. Lai, Y. T, Huang; Life Sciences; Vol. 57; Issue 13; 1995
- http://www.iscid.org/encyclopedia/Acetylcholine
- ↑ 1.0 1.1 Images taken from http://www.neurosci.pharm.utoledo.edu/MBC3320/acetylcholine.htm
- ↑ Zelder, F. H. F. (2006). "Cavitand templated catalysis of acetylcholine." Chemical communications(7): 753.
- ↑ JW Ye, JX Cai, LM Wang, XC Tang - J Pharmacol Exp Ther, 1999 - ASPET
Awc106 13:52, 28 November 2007 (UTC)