Acc2518
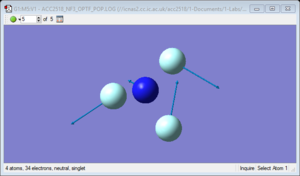
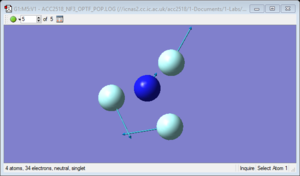
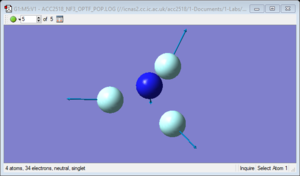
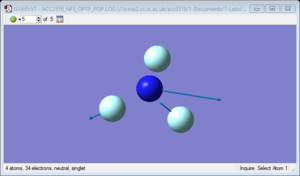
NH3 molecule
Optimisation data
| Calculation method | RB3LYP |
| Basis set | 6-31G(d.p) |
| Final energy E(RB3LYP) | -56.55776873 (au) |
| RMS gradient | 0.00000485 (au) |
| Point group | C3V |
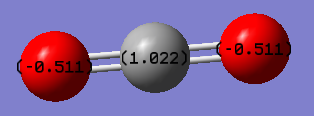
| Bond length (N-H) | 1.02 (Å) |
| Bond angle (H-N-H) | 106(°) |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 molecule |
Optimised NH3 .log file (acc2518)
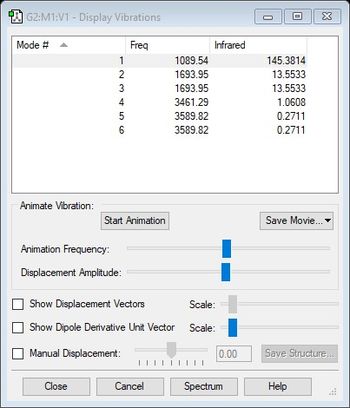
Vibrations
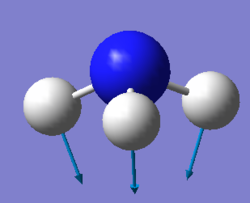
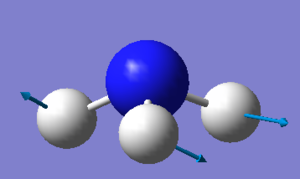
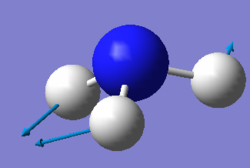
Using the 3N-6 rule, where N represents the number of atoms in a molecule, it is expected that ammonia will have six vibrational modes as shown above. There are two pairs of degenerate modes with a frequency of 1694 (cm-1) and 3590 (cm-1). The modes with a frequency of 1090 (cm-1) and 1694 (cm-1) correspond to bending vibrations and the modes with a frequency 3461 (cm-1) and 3590 (cm-1) correspond to bond stretch vibrations. The bond stretch vibration at 3461 (cm-1) is highly symmetric whilst the stretches with a frequency of 3590 (cm-1) are asymmetric. The bending vibration with a frequency of 1090 (cm-1) is known as the umbrella mode. Three bands would be expected to be seen with the two stretches at 3590 (cm-1) being too low in intensity. The bends with a frequency of 1694 (cm-1) are degenerate and only one band will be observed between the two. Hence the three observed bands would be at 1090 (cm-1), 1694 (cm-1) and 3461 (cm-1).
Charge analysis
Nitrogen has a greater electronegativity than hydrogen and hence there is greater electron density distributed around the N atom in ammonia relative to hydrogen. This explains the negative charge on N and the positive charge on H.
N2 molecule
Optimisation data
| Calculation method | RB3LYP |
| Basis set | 6-31G(d.p) |
| Final energy E(RB3LYP) | -109.52412868 (au) |
| RMS gradient | 0.00000060 (au) |
| Point group | D∞h |
| Bond length (N-N) | 1.11 (Å) |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Nitrogen molecule |
Optimised N2 .log file (acc2518)
Vibrations
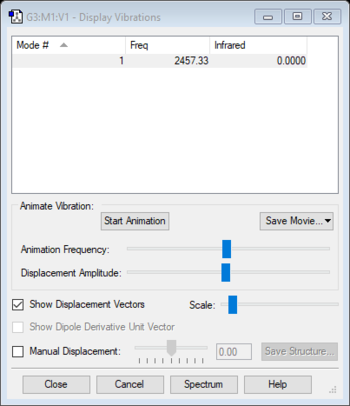
| Wavenumber (cm-1) | 2457 |
| Symmetry | SGG |
| Intensity (arbitrary units) | 0 |
| Image | 
|
Using the 3N-5 rule for linear molecules, Nitrogen can be expected to have one vibrational mode but this mode has an IR intensity of zero since Nitrogen molecules are IR inactive. This is because they have no overall dipole.
Charge analysis
Both nitrogen atoms have the same electronegativity so the charge is neutral on both atoms.
CCDC search
Unique identifier: VAMQAE
Deposition number: 1530121
The complex linked above contains nitrogen coordinated to cobalt with a N-N bond distance of 1.08 Å which is slightly shorter than the optimised N-N bond length, displayed above, of 1.11 Å (2.d.p). During the optimisation process for nitrogen, assumptions have to be made for the computational process to be carried out. Each assumption made will increase the uncertainty in measurable quantities such as the bond length. Hence, the computationally determined bond length from the optimisation is a model and may not fully reflect the true bond length or the bond length determined by a computational program considering slightly different assumptions.
It should also be considered that the optimisation gave a prediction for the N-N bond length in a nitrogen molecule whereas the molecule linked above contains a nitrogen molecule that is coordinated into a transition metal complex. A lone pair of electrons on one of the nitrogen atoms has been donated to a cobalt ion, this draws electron density away from the donating nitrogen atom and would therefore slightly increase the positive charge on this atom. This may cause the donating nitrogen atom to attract the electrons in the N-N bond more strongly which would then explain the shorter bond in the transition metal complex compared to the nitrogen molecule.
H2 molecule
Optimisation data
| Calculation method | RB3LYP |
| Basis set | 6-31G(d.p) |
| Final energy E(RB3LYP) | -1.17853936 (au) |
| RMS gradient | 0.00000017 (au) |
| Point group | D∞h |
| Bond length (H-H) | 0.74 (Å) |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Hydrogen molecule |
Optimised H2 .log file (acc2518)
Vibrations
| Wavenumber (cm-1) | 4466 |
| Symmetry | SGG |
| Intensity (arbitrary units) | 0 |
| Image | 
|
Using the 3N-5 rule for linear molecules, hydrogen can be expected to have one vibrational mode but this mode has an IR intensity of zero since hydrogen molecules, like nitrogen molecules, are IR inactive.
Charge analysis
Both hydrogen atoms have the same electronegativity so the charge is neutral on both atoms.
The Haber-Bosch process
N2 + 3H2 -> 2NH3
E(NH3)=-56.55776873
2*E(NH3) =2*(-56.55776873)=-113.11553746
E(N2) =-109.52412868
E(H2)=-1.17853936
3*E(H2) =3*(-1.17853936) =-3.53561808
ΔE= 2*E(NH3) -[ E(N2) + 3*E(H2) ]= -0.05579069 ≈ -0.05579 au (5.d.p)
ΔE=-146.5 kJ/mol (1.d.p)
ΔE is negative which indicates that the gaseous reactants are less stable than the ammonia product since the reactants are at a higher energy than the product. Although this process is not necessarily entropically favourable with four reactant gaseous molecules forming two gaseous product molecules, the reaction has a significantly negative enthalpy change which reflects the result calculated above.
Project molecule (NF3)
Optimisation data
| Calculation method | RB3LYP |
| Basis set | 6-31G(d.p) |
| Final energy E(RB3LYP) | -354.07131058 (au) |
| RMS gradient | 0.00010256 (au) |
| Point group | C3V |
| Bond length (N-F) | 1.38 (Å) |
| Bond angle (F-N-F) | 102(°) |
Item Value Threshold Converged? Maximum Force 0.000164 0.000450 YES RMS Force 0.000108 0.000300 YES Maximum Displacement 0.000612 0.001800 YES RMS Displacement 0.000296 0.001200 YES
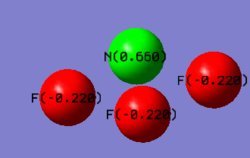
NF3 molecule |
Optimised NF3 .log file (acc2518)
Vibrations
Nitrogen trifluoride has the same number of atoms as ammonia and so six vibrational modes can be predicted from the 3N-6 rule. There are also two pairs of degenerate modes with frequencies of 482 (cm-1) and 930 (cm-1). The modes with a frequency of 482 (cm-1) and 644(cm-1) correspond to bending vibrational modes and the modes with a frequency of 930 (cm-1) and 1062 (cm-1) correspond to stretching vibrational modes. The mode with a frequency of 1062 (cm-1) represents a symmetric stretch whilst the modes with a frequency of 930 (cm-1) represent asymmetric stretches. In contrast to ammonia, the highest frequency bond stretch of nitrogen trifluoride is the symmetric stretch.
Three bands can be expected in the NF3 IR spectrum at frequencies of 644 (cm-1) 930 (cm-1) and 1062 (cm-1). The bends at 482 (cm-1) will be too low in frequency to appear in the spectrum which starts at a frequency of 500 (cm-1). The stretches at 930 (cm-1) are degenerate and hence will only display one band between them. The band as 644 (cm-1) may not be clearly visible due its relative low intensity of 3 arbitrary units.
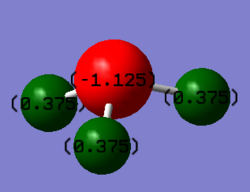
Charge analysis
Fluorine has a greater electronegativity that nitrogen and therefore there is greater electron density distributed around the three fluorine atoms compared to the nitrogen atom. The three fluorine atoms are treated as equivalent which means that the magnitude of the positive charge on nitrogen is three times the magnitude of the negative charge on a single fluorine atom.
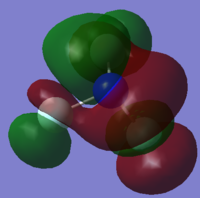
Molecular orbitals (MO)
The nitrogen atom contributes 7 electrons and each fluorine atom contributes 9 electrons which means that nitrogen trifluoride contains 34 electrons in total and hence 17 electron pairs. In the table below, a molecular orbital number of 1 would denote the molecular orbital that is deepest in energy. Therefore, orbital 17 represents the HOMO and orbital 18 represents the LUMO. All of the molecular orbitals shown below are occupied with the exception of the LUMO.
Bonus Molecule (CO2)
Optimisation data
| Calculation method | RB3LYP |
| Basis set | 6-31G(d.p) |
| Final energy E(RB3LYP) | -188.58093945 (au) |
| RMS gradient | 0.00001154 (au) |
| Point group | D∞h |
| Bond length (C-O) | 1.17 (Å) |
| Bond angle (C-O-C) | 180 (°) |
Item Value Threshold Converged? Maximum Force 0.000024 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.000021 0.001800 YES RMS Displacement 0.000015 0.001200 YES
Carbon dioxide molecule |
Optimised CO2 .log file (acc2518)
Vibrations
| Wavenumber (cm-1) | 640 | 640 |
| Symmetry | PIU | PIU |
| Intensity (arbitrary units) | 31 | 31 |
| Image | 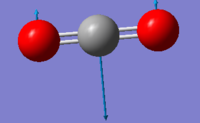 |
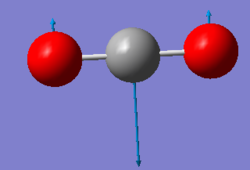
|
| Wavenumber (cm-1) | 1372 | 2436 |
| Symmetry | SGG | SGU |
| Intensity (arbitrary units) | 0 | 546 |
| Image | 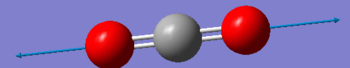 |
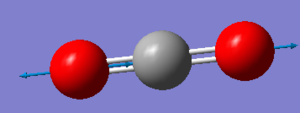
|
Using the 3N-5 rule for linear molecules, carbon dioxide can be expected to have 4 vibrational modes. The modes with a frequency of 640 cm-1 correspond to bends and the modes with frequencies of 1372 cm-1 and 2436 cm-1 correspond to stretches. The two modes with a frequency of 640 cm-1 are degenerate and will only produce one IR band between them. The stretch with a frequency of 1372 will not produce an IR band as the stretch is symmetric and has no overall dipole. The asymmetric stretch at 2436 cm-1 will also produce an IR band to give two IR bands overall.
Charge analysis
Oxygen has a greater electronegativity than carbon which explains the negative charge on each oxygen atom and the positive charge on the central carbon atom.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4.5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES, overall a very good wiki with detailed explanations and a nice layout, well done! The explanation of the charges is good and most of the MO discussion is good. In MO17 however the main contribution to the N atom is of s rather than p character. Overall this MO is antibonding between the F and N atoms with pi bonding amongst the F atoms.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
You calculated CO2 well done!
Do some deeper analysis on your results so far