5446WMN
Felix Introduction to Molecular Modelling - Dr. Hunt
Optimisation, Vibrations and Charges, Reactions and Orbitals
After being introduced to ChemWiki and GaussView, we learnt the basics of optimisation, using NH3 as our test molecule.
NH3 Optimisation
| Molecule | NH3 |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p.) |
| Final Energy E(RB3LYP) (au) | -56.557769 |
| RMS Gradient | 0.00000485 |
| Point Group | C3V |
| Optimised Bond Distance (Å) | 1.01798 |
| Optimised Bond Angle (°) | 105.741 |
The following is an excerpt from the 'real' output, which is the file that Gaussian generates. The fact that the the Maximum force of any of the items does not exceed 0.00045 au and the RMS does not exceed 0.0003 au.
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Ammonia Molecule |
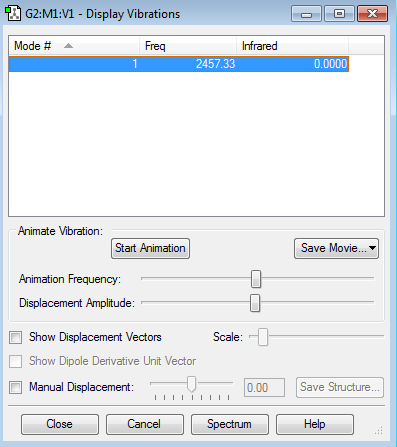
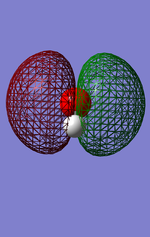
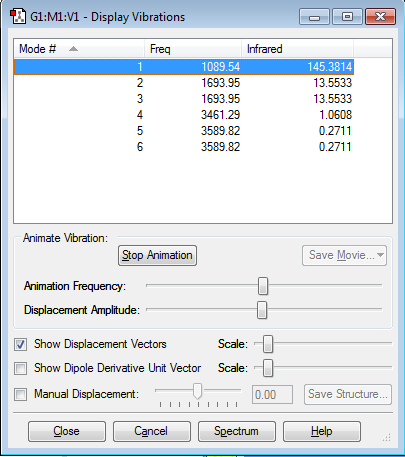 This screenshot shows the vibrational modes of the NH3 molecule calculated by GuassView.
File:FD915 HUNT MOLMOD NH3 OPT POP.LOG
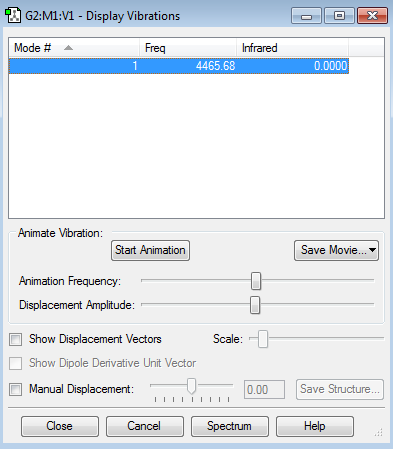
This screenshot shows the vibrational modes of the NH3 molecule calculated by GuassView.
File:FD915 HUNT MOLMOD NH3 OPT POP.LOG
| How many modes do you expect from the 3N-6 rule? | 6 |
|---|---|
| Which modes are degenerate (ie have the same energy)? | Modes 2/3 and modes 5/6 are degenerate |
| Which modes are "bending" vibrations and which are "bond stretch" vibrations? | Modes 1,2,3 are bending, and modes 4,5,6 are stretching. |
| Which modes are highly symmetric? | Modes 1 and 4 are highly symmetric |
| One mode is known as the "umbrella" mode, which one is this? | The first mode |
| How many bands would you expect to see in an experimental spectrum of gaseous ammonia? | 2, due to the low intensity of modes 4,5,6. |
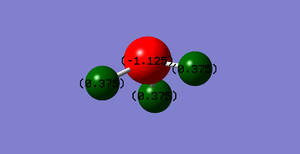
This thumbnail shows the nitrogen of ammonia in red to highlight its negative charge of -1.125, and the hydrogens in green with a positive charge of 0.375.
N2 Optimisation:
| Molecule | N2 |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p.) |
| Final Energy E(RB3LYP) (au) | -109.5241 |
| RMS Gradient | 0.0000006 |
| Point Group | D∞h |
| Optimised Bond Distance (Å) | 1.1055 |
| Optimised Bond Angle (°) | 180 |
Again, the values for the Maximum Displacement and the RMS show that the molecule has been properly optimised.
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
The table shows that the molecule has only one vibrational mode.
Nitrogen Molecule |
File:FD915 NITROGEN OPTIMISATION.LOG
H2 Optimisation:
| Molecule | H2 |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p.) |
| Final Energy E(RB3LYP) (au) | -1.17854 |
| RMS Gradient | 0.00000017 |
| Point Group | D∞h |
| Optimised Bond Distance (Å) | 0.74279 |
| Optimised Bond Angle (°) | 180 |
Note the especially low value for the final energy of the hydrogen molecule.
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES

Hydrogen Molecule |
File:FD915 HYDROGEN OPTIMISATION.LOG
Energies Involved in Production of NH3 (au):
| E(NH3)= -56.55776873 |
|---|
| 2*E(NH3)= -113.1155375 |
| E(N2)=-109.52412868 |
| E(H2)=-1.17853936 |
| 3*E(H2)= -3.53561808 |
| ΔE=2*E(NH3)-[E(N2)+3*E(H2)] = -0.0557907 |
| In kJ/mol = -146.4784829 |
The ammonia is more stable than the gaseous reactants, due to the large negative energy value.
My Own Molecule
| Molecule | H2O |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p.) |
| Final Energy E(RB3LYP) (au) | --76.41973740 |
| RMS Gradient | 0.000046276 |
| Point Group | C2V |
| Optimised Bond Distance (Å) | 0.96522 |
| Optimised Bond Angle (°) | 103.745 |
The value for the Maximum Force/RMS show that the molecule has been optimised properly, and that the angle for the water molecular is apparently 103.75°, whereas I thought the value was supposed to be around 104.5°.
Item Value Threshold Converged? Maximum Force 0.000099 0.000450 YES RMS Force 0.000081 0.000300 YES Maximum Displacement 0.000115 0.001800 YES RMS Displacement 0.000120 0.001200 YES
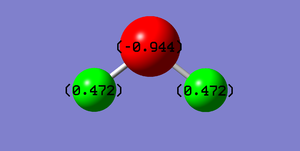
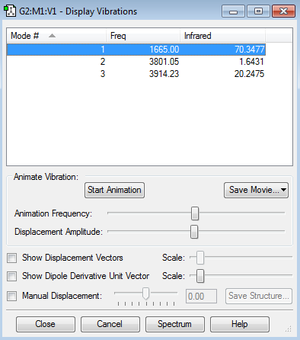
Water Molecule |