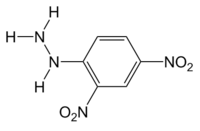
2,4-Dinitrophenylhydrazine
| 2,4-Dinitrophenylhydrazine | ||||
|---|---|---|---|---|
| ||||
| General | ||||
| Systematic name | 2,4-Dinitrophenylhydrazine | |||
| Other names | 2,4-DNPH, Brady's Reagent | |||
| Molecular formula | C6H6N4O4 | |||
| SMILES | [H]N([H])N([H])C1=CC=C([N+]
[O-])=O)C=C1[N+]([O-])=O | |||
| Molar mass | 198.14 | |||
| Appearance | wet, red or orange, crystalline powder | |||
| CAS number | 119-26-6 | |||
| Properties | ||||
| Solubility in water | slight solubility in water | |||
| Melting point | 200-202°C (473-475K) decomposes | |||
| Hazards | ||||
| Main hazards | 
potential carcinogen | |||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | ||||
This article is about the compound 2,4-Dinitrophenylhydrazine, otherwise known as 2,4-DNPH or Brady's Reagent. It is a substituted hydrazine (NH2NH2) and is most commonly used as a qualitative test for the presence of the carbonyl functional group (C=O).
Synthesis
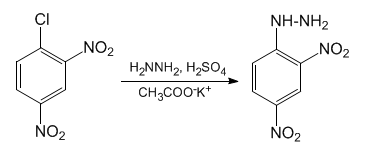
2,4-DNPH can be synthesized via the electrophilic substitution reaction of hydrazine with 2,4-dinitrochlorobenzene. This synthesis was carried out by C. F. H. Allen in 1943[1]. The reaction scheme of this reaction is shown below.
Alternative Methods of Synthesis
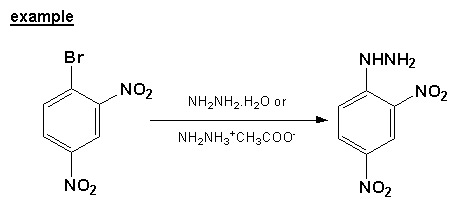
Besides using hydrazine, the synthesis of 2,4-dinitrophenylhydrazine has also been carried out using hydrazine hydrate[2] (OH2-H2N-NH2) and hydrazine acetate (NH2NH2)(CH3COOH). On top of that, the 2,4-dinitrochlorobenzene reagent can be replaced with its respective bromine derivative.
Reaction with Carbonyl Compounds
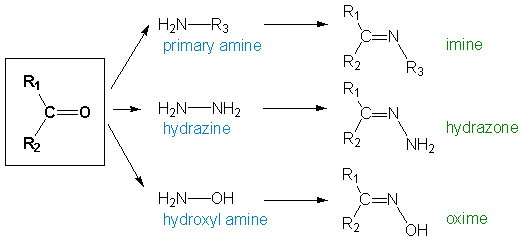
2,4-dinitrophenylhydrazine, having a nitrogen atom with lone pairs, is an effective nucleophile. On the other hand, carbonyl compounds, with the polarized carbon-oxygen double bonds, have electrophilic carbon centres. As such, it is no surprise that 2,4-dinitrophenylhydrazine will react readily with carbonyl compounds in a condensation reaction, more specifically known as the Brady's Reaction. The overall reaction scheme and mechanism of this reaction is shown below.
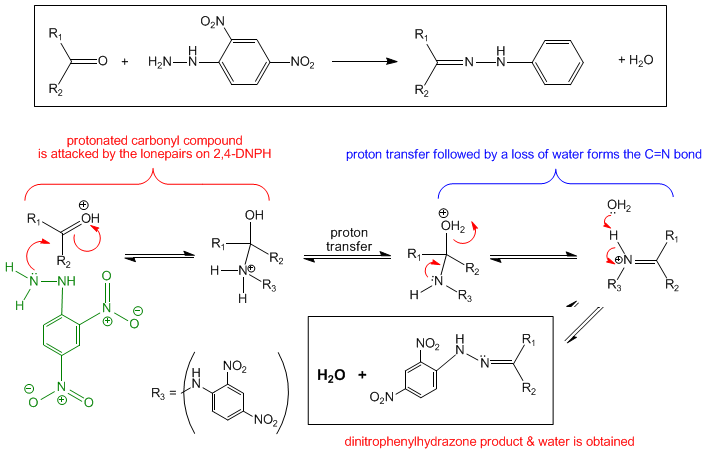
In chemical experiments, the solid 2,4-DNPH is first dissolved in a methanol/sulphuric acid solvent to give a yellow solution of 2,4-DNPH. This solution is what we call Brady's Reagent. A few drops of carbonyl compound is then added to Brady's Regeant and the reaction will proceed smoothly with no need for heating or any other reagents.
The products obtained from this reaction are water and an organic compound which is known as a dinitrophenylhydrazone. In general, a hydrazone is a class of organic compound which contains the C=N double bond, with the general formula of R2C=NR2. As water is eliminated in the Brady's Reaction, we can see why this reaction is also considered a condensation reaction. In terms of mechanism, this reaction proceeds via a nucleophilic addition-elimination mechanism. The 2,4-DNPH first adds across the carbon-oxygen double bond to form an intermediate, which then gives the final product upon the elimination of a water molecule.

Using the Brady's Reaction
As mentioned above, 2,4-dinitrophenylhydrazine is mainly used as a positive test for the presence of a carbonyl functional group. The addition of Brady's Reagent to a compound containing the carbonyl functional group will result in the formation of a dinitrophenylhydrazone, which is actually an orange or yellow insoluble solid. As such, with just a simple addition of a few drops of 2,4-DNPH, we are able to tell whether a carbon-oxygen double bond is present, by observing the formation (or absence) of a yellow/orange precipitate. As other functional groups will not react with the hydrazine, Brady's reagent is one of the most useful substances that enable chemists to distinguish aldehydes and ketones from other functional groups.
Dinitrophenylhydrazones
Besides just testing for the presence of the carbon-oxygen double bond, the Brady's Reaction can also be used to identify the specific aldehyde or ketone. The precipitate obtained is usually filtered, washed (for example, with methanol) and then recrystallised from a suitable solvent. The solvent used will vary depending on the nature of the carbonyl compound involved. For example, the hydrazones from smaller aldehydes and ketones can be recrystallised from a mixture of ethanol and water.
The 2,4-DNPH derivatives obtained from the reaction of 2,4-DNPH with the various carbonyl compounds have distinct characteristic colours and melting points. There are data tables which show the melting points of the derivatives of common aldehydes and ketones. As such, by finding the melting point of the crystals obtained after recrystallisation, it is possible to identify the carbonyl compound by comparing it with tables of known melting points to find out which carbonyl compound you are likely to have. Some examples of aldehydes and ketones and the melting points of their respective hydrazones are given in the table below.
| Ketone | BP/°C | MP of 2,4-DNP derivative/°C | Aldehyde | BP/°C | MP of 2,4-DNP derivative/°C | |||
| Acetone (Propanone) | 56 | 128 | Propanal | 48 | 150 | |||
| Butanone | 82 | 117 | Butanal | 75 | 123 | |||
| 3-pentanone | 102 | 156 | Pentanal | 103 | 98 | |||
| Cyclohexanone | 156 | 162 | Hexanal | 131 | 104 | |||
| 1-Phenyl-2-propanone | 216 | 191 | Benzaldehyde | 179 | 237 |
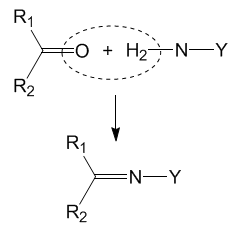
Similar Addition-Elimination Reactions
The reaction between 2,4-dinitrophenylhydrazine and carbonyl compounds is actually a very common reaction. It can be observed from the reaction scheme that only the NH2 part of the molecule reacts and the rest remain unchanged. If the NH2 group is attached to other things, similar reactions can also occur via the same mechanism. Some examples of such reactions are shown below.[4] In each reaction, water is lost (condensation process) and a C=N double bond is formed, giving different functional groups in the final products. These reactions are very useful for functional group interconversions.
Spectra
References
- ↑ C.F.H Allen, Organic Syntheses, 1943, 2, p228.
- ↑ O.L. Brady;G.V. Elsmie, Analyst, 1926, 51, p77
- ↑ http://acpcommunity.acp.edu/facultystaff/genchem/GC1/lab/qual/dnp.jpg
- ↑ MSU Department of Chemistry, Virtual Textbook of Organic Chemistry, http://www.cem.msu.edu/%7Ereusch/VirtualText/aldket1.htm#rx1
Contributed by: Seg06 - GOH Astee (Imperial College Chemistry Year 2)