1styearassignmentdp513
Computational analysis of NH3 and hydrogen peroxide
This page contains a report constituting part of the first year physical chemistry lab course. It aims to provide account of optimization calculations of a range of molecules using gaussian and report on the key features of those optimized structures such as energies of equilibrium states, vibrational modes and their frequencies and visualization of molecular orbitals.
NH3
File Name = FRIST(3)NH3OPTIMIZATION2 Molecule name = NH3 Calculation Method = RB3LYP Basis Set = 6-31G(d,p) E(RB3LYP) = -56.55776873 a.u. RMS Gradient Norm = 0.00000485 a.u. Point Group = C3V
NH3 molecule |
Questions about molecular vibrations
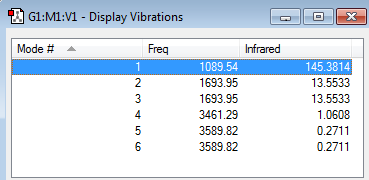
- - 6 vibrations are expected as the molecule is formed from 4 atoms.
- - Scisoring of different N-H bonds at 1693 frequency units in and two assymetric bond stretches at 3589 units, in one of which stretch of the two N-H bonds while position of the other remains fixed, in the second mode all three bonds stretch. Similar pattern occurs with scissoring.
- - Mode numbers 1,2,3 are bending modes while 4,5,6 are stretches.
- - symmetric stretch at 3461 units.
- - symmetric bend at 1089 units.
- - 4.
Atomic charges
According to the NBO analysis atomic charge on N is 1.125e and on H the value is -0.375e
Reaction energies
After running an optimization of N2 and H2 the following parameters were determined.
| Property | N2 | H2 |
|---|---|---|
| File Name | n2optimizationdp513 | H2OPTIMIZATION2DP513 |
| Energy | -109.52412868 | -1.17853936 |
| RMS Gradient Norm | 0.00000060 | 0.00000204 |
| Frequency | 2457 | 4466 |
H2
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.167770D-13
Optimization completed.
N2
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.383675D-13
Optimization completed.
Calculation
- E(NH3)=-56.55776873
- 2*E(NH3)=-113.1155375
- E(N2)=-109.5241287
- E(H2)=-1.17853936
- 3*E(H2)= -3.53561808
- ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -146.4784828 Kjmol-1
MOs
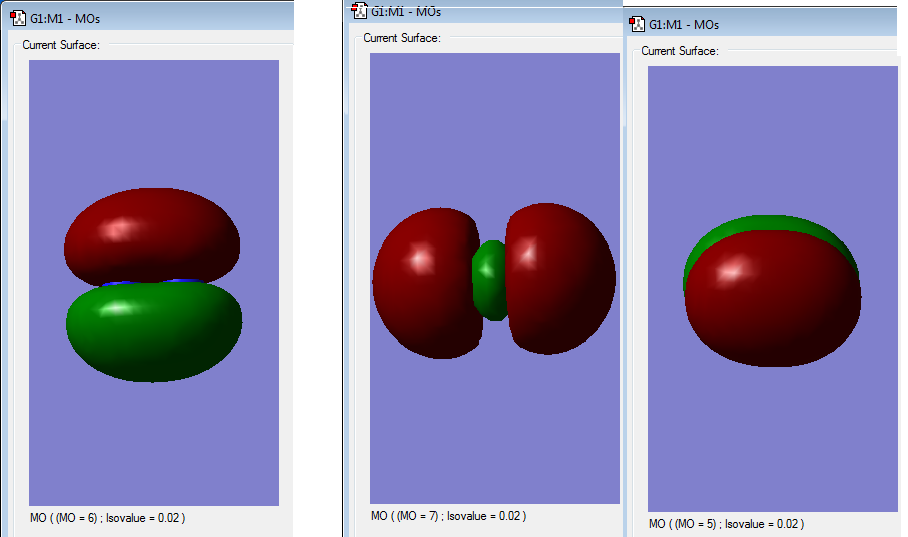
Below are images of the three highest (with HOMO being the MO 7 ) occupied molecular orbitals of N2. There is apparent in phase orbital overlap which shows that all three highest occupied molecular orbitals are bonding.
Reference gaussveiwfiles
File:FRIST(3)NH3OPTIMIZATION2.LOG
Media:H2OPTIMIZATION2DP513.LOG
Hydrogen peroxide
Intro
Molecule under investigation in the independent part of the lab was chosen to be O2H2. The reason + a historical fact(?) was that hydrogen peroxide was used as torpedo propellent in the 1950-60. Then it was decommissioned in most fleets around the world apart from ussr and potentially even there. In 2000 Russian navy dug up those old torpedoes and used them during naval exercises as a cost saving measure. Result - a blown up submarine resting on the oceans floor(partly lifted though) and a few dozen dead personnel. Peroxide leaked and rapid decomposition started catalysed by the rust on the torpedo launch tube resulting in an explosion.
Optimization
Hydrogen peroxide optimization
File Name HYDROGENPEROXIDEOPTIMIZATIONDP513
File Type .log
Calculation Type FREQ
Calculation Method RB3LYP
Basis Set 6-31G(d,p)
Charge 0
Spin Singlet
E(RB3LYP) -151.54319153 a.u.
RMS Gradient Norm 0.00001203 a.u.
Dipole Moment 1.7585 Debye
Point Group C1
Item section of the output file
Item Value Threshold Converged?
Maximum Force 0.000033 0.000450 YES
RMS Force 0.000017 0.000300 YES
Maximum Displacement 0.000432 0.001800 YES
RMS Displacement 0.000375 0.001200 YES
Predicted change in Energy=-6.479291D-09
Optimization completed.
Jmol
Hydrogen Peroxide |
Charges
Optimized structure was found to have charge of 0.478e on oxygen atoms and -0.478 on hydrogens
Vibrations
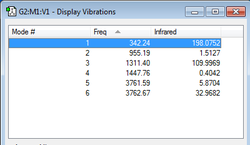
2nd vibrational mode is the o-o bond stretch with a very low frequency value of 955 indicating weakness of this bond hence easy decomposition of hydrogen peroxide.
Modes 5 and 6 are degenerate (within 1 unit of frequency) symmetric and asymmetric stretches of the O-H bonds.
MO
| MO Picture | MO Energy | Bonding/antibonding |
|---|---|---|
|
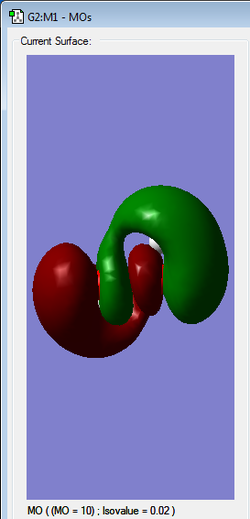
LUMO | antibonding |
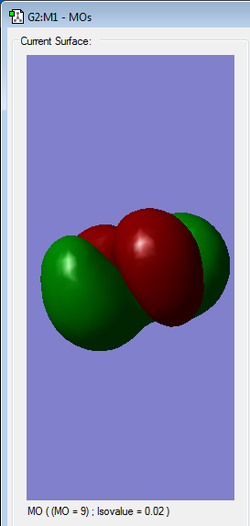
|
HOMO | Antibonding |
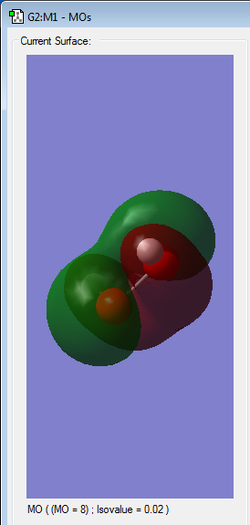
|
2nd HOMO | antibonding |
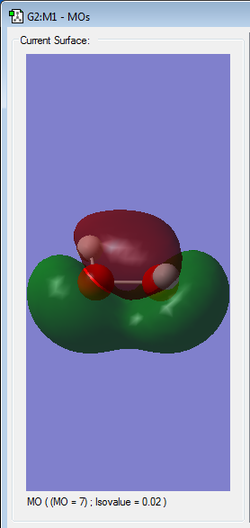
|
3rd HOMO | perhaps bonding as no node in between atom groups(?) |
Orbitals in the HOMO/LUMO region are high in energy explaining the instability of the molecule.