0MW7FY8
Jacob Forrest
Optimisation of NH3
Molecule - NH3
the method: B3LYP
the basis set: 6-31G(d,p)
final energy E(RB3LYP) in atomic units: -56.55776873 au
point group : C3v
Bond length : 1.01798 au
H-N-H bond angle : 105.745 degrees
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000014 0.001800 YES
RMS Displacement 0.000009 0.001200 YES
Ammonia |
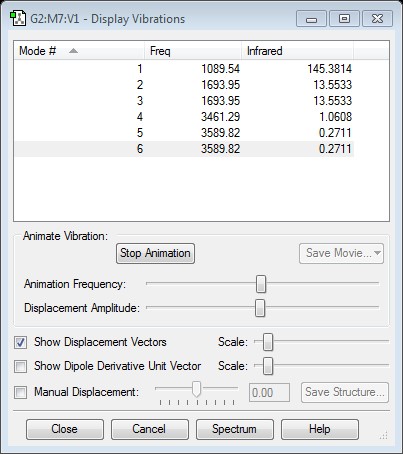
NH3 bond vibrations
how many modes do you expect from the 3N-6 rule?
6 because NH3 has 6 modes of vibration
which modes are degenerate (ie have the same energy)?
2+3, 5+6 , They are both the two asymmetric stretches(5+6) and bends(2+3)
which modes are "bending" vibrations and which are "bond stretch" vibrations?
1,2,3 then 4,5,6
which mode is highly symmetric?
4 because the symmetric stretches do not at all affect the shape or dipoles of the the molecule
one mode is known as the "umbrella" mode, which one is this?
1 because the symmetric stretching animation looks similar to the opening and closing of an umbrella
how many bands would you expect to see in an experimental spectrum of gaseous ammonia?
4 because there are 4 vibration modes that create imbalanced dipoles (2,3,5,6)
Charge distribution in NH3
It is expected for the Nitrogen to have a negative charge as it is more electronegative than hydrogen and therefore will draw the majority of electron density of the molecule to itself this in turn means the hydrogen is expected to be positive and for all of the hydrogen atoms to have the same magnitude of charge as they are all equally electronegative and equally distanced from the nitrogen atom.
Nitrogen charge = -1.125
Hydrogen charge = 0.375
Molecule - N2
Molecule : N2
the method: B3LYP
the basis set: 6-31G(d,p)
final energy E(RB3LYP) in atomic units: -109.52412868 au
point group : D∞h
Bond length : 1.10550 A
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Vibrations - #Mode:1 // Freq: 2457.33cm-1
Molecule - H2
Molecule : H2
the method: B3LYP
the basis set: 6-31G(d,p)
final energy E(RB3LYP) in atomic units: -1.17835689 au
point group : D∞h
Bond length : 0.74279 A
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Vibrations - #Mode:1 // Freq: 4465.68cm-1
Reaction energy of Haber-Bosch process
E(NH3)= -56.55776873 au 2*E(NH3)= -113.11553746 au E(N2)= -109.52412868 au E(H2)= -1.17835689 au 3*E(H2)= -3.53507067 au ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05633811 au Therefore in kj/mol = -147.92 (2 d.p)
So the ammonia product is more stable as the forming of the ammonia releases more energy than the separation of the N2 amd H2 molecules; the reaction is exothermic. However this does not mean that N-H bonds individually more stable than the reactants but the overall energy and proportionally the stability of the N-H bonds formed is greater than the energy required to break the N2 and H2 bonds for the formation of the ammonia.
ClF3 optimisation
Vibrations and charges
mode freq(cm-1)
1 -43.17
2 -43.16
3 416.55
4 559.35
5 610.87
6 610.87
Fluorine charge = -0.396 Chlorine charge = 1.188