01532430
NH3
test molecule |

| More Info | |
|---|---|
| N-H Bond Distance: | 1.01798Å |
| H-N-H Bond Angle: | 105.741° |
| Calculation Method: | RB3LYP |
| Basis Set: | 6-31G(d,p) |
| Final Energy: | -56.55776873 |
| RMS gradient: | 0.00000485 |
| Point Group | C3V |
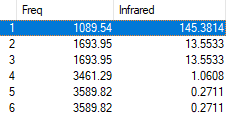
Symmetry of Vibrations
| Symmetry | A1 | E |
|---|---|---|
| Wavenumber (cm-1) | 3461 | 3590 |
| Intensity | 145 | 14 |
 |
 |
Charges on Atoms
Charge on each atom: Nitrogen=-1.125, Hydrogen=0.375
Questions:
1) how many modes do you expect from the 3N-6 rule? 6
2) which modes are degenerate (ie have the same energy? Modes 2 and 3, Modes 5 and 6, are degenerate
3) which modes are "bending" vibrations and which are "bond stretch" vibrations? 1,2,3 are bond vibrations while 4,5,6 are bond stretches
4) which mode is highly symmetric? 4
5) one mode is known as the "umbrella" mode, which one is this? 1
6) how many bands would you expect to see in an experimental spectrum of gaseous ammonia? 4
N2
test molecule |
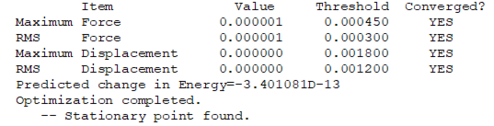
| More Info | |
|---|---|
| N-N Triple bond distance: | 1.10550 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -109.52412868 |
| RMS gradient | 0.00000060 |
| Point Group | D*H |
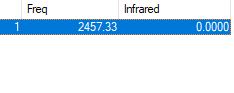
Symmetry of Vibrations
| Symmetry | SGG |
|---|---|
| Wavenumber (cm-1) | 2457 |
| Intensity | 0 |
 |
H2
test molecule |


| More Info | |
|---|---|
| H-H Bond Distance | 0.74279 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -1.17853936 |
| RMS gradient | 0.00000017 |
| Point Group | D*H |
Symmetry of Vibrations
| Symmetry | SGG |
|---|---|
| Wavenumber (cm-1) | 4466 |
| Intensity | 0 |
 |
Haber-Bosch Process
Measured in a.u.
- E(NH3)= -56.55777
- 2*E(NH3)= -113.11554
- E(N2)= -109.52413
- E(H2)= -1.17854
- 3*E(H2)= -3.53562
- ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579a.u = -146KJmol-1
Mono-metallic TM Complex
test molecule |
CCDC Unique Identifier: KECVUJ
H-H Bond length of TM complex: 0.814577
H-H Bond length of hydrogen molecule: 0.74279
The above structure reported by Ricci et al.[1]
//place
Q. Why are the bond lengths different?
A. The method of working out the bond length is RB3LYP. This method is not the most sophisticated and so will lead to a potentially inaccurate bond length. Not only is the method not highly complex but the hardware being used isn't powerful enough to carry out the calculations required to find an accurate value of either bond length. Despite this for both bond length calculations, the same method was used. Therefore, using the RB3LYP method, within this complex, the hydrogen bond is longer then that of the bond of the hydrogen molecule. This is due to the electrons in the HOMO bonding orbital of the hydrogen molecule bonding to the d orbital of the metal complex, as well as some of the electron density now being in the LOMO orbital of the hydrogen MO. This overall makes the bond between the hydrogen atoms weaker which is shown by the longer bond.
SH2
test molecule |

| More Info | |
|---|---|
| S-H Bond Distance | 1.31000 |
| H-S-H Bond Angle | 92.250 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -399.39162414 |
| RMS gradient | 0.00012068 |
| Point Group | C2V |
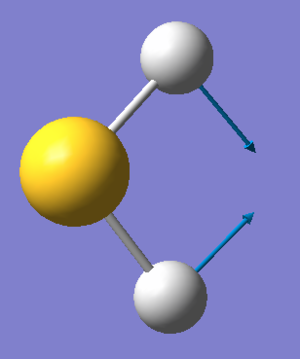
Symmetry of Vibrations
| Symmetry | A1 | A1 |
|---|---|---|
| Wavenumber (cm-1) | 1224 | 2692 |
| Intensity | 4.922 | 6.735 |
 |
 |
Molecular Orbitals of SH2
References
- ↑ Single-crystal x-ray and neutron diffraction studies of an .eta.2-dihydrogen transition-metal complex: trans-[Fe(.eta.2-H2)(H)(PPh2CH2CH2PPh2)2]BPh4 John S. Ricci, Thomas F. Koetzle, Maria T. Bautista, Theresa M. Hofstede, Robert H. Morris, and Jeffery F. Sawyer Journal of the American Chemical Society 1989 111 (24), 8823-8827 DOI: 10.1021/ja00206a009.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 0.5/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES overall it looks good well done!
Do you effectively use tables, figures and subheadings to communicate your work?
YES however you have left the caption for your jmols as "test molecule" for all of them. Also just one of your subheadings is bold for some reason which is inconsistent and confusing to a reader. "Measured in a.u." is also not a useful subheading!
NH3 0/1
Have you completed the calculation and given a link to the file?
NO - there is no link to the file present. (It is included in the jmol but this does not count as a direct link.)
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
The correct charges are present and you have correctly answered most of the questions on the vibrations, well done! However you would expect only 2 visible peaks in the spectrum as peaks 4, 5 and 6 are very low in intensity.
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
There is no link to the files present. (It is included in the jmol but this does not count as a direct link.)
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES - the section about the bonding between the complex and the H2 molecule is very good well done!
Haber-Bosch reaction energy calculation 0.5/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
No - you didn't remember to answer the question.
Your choice of small molecule 2/5
Have you completed the calculation and included all relevant information?
YES There is no link to the files present. (It is included in the jmol but this does not count as a direct link.)
Have you added information about MOs and charges on atoms?
You haven't included any information on the charges.
You have included pictures of several relevant MOs in a nicely presented table - well done!
The description of MO2 is correct.
MO3 is one of the three S 2p AOs.
MO9 is the one of the 3 S 3p AOs, there is no overlap with the H 1s AOs.
MO6 is S 3s and H 1s bonding, you are correct. It has a low energy, not a high energy.
MO11 looks like S 3s and H 1s anti bonding. There is no S 3p AO here.
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far
You didn't do any extra work for the independence mark.