01497033
NH3
| Optimised N-H bond length (Å) | 1.01798 |
| Optimised H-N-H bond angle (°) | 105.741 |
Due to a compromise between accuracy and speed of calculation, these values will have some uncertainty. Bond length is accurate to ≈ 0.01Å; bond angle is accurate to ≈ 1°
| Molecule | NH3 |
| Calculation method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy E(RB3LYP) (a.u.) | -56.55776873 |
| RMS gradient (a.u.) | 0.00000485 |
| Point group | C3V |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
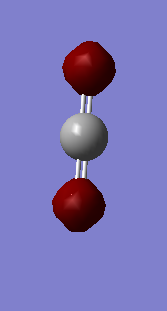
Ammonia molecule |
The optimisation file is linked to here
Vibrational analysis
| Wavenumber (cm-1) | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| IR Intensity (KM/mol) | 145 | 14 | 14 | 1 | 0 | 0 |
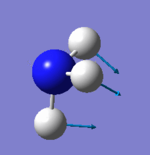 |
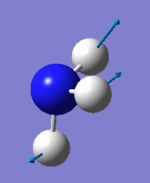 |
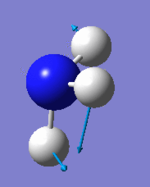 |
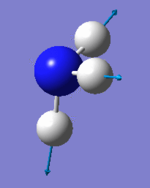 |
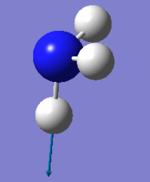 |
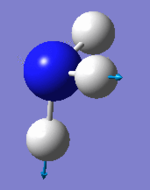
|
Frequency analysis
how many modes do you expect from the 3N-6 rule?
(3*4) – 6 = 6 modes
Which modes are degenerate (ie have the same energy)?
Both E symmetries (1694 cm-1 and 3590 cm-1
Which modes are “bending” vibrations and which are “bond stretch” vibrations?
1090 cm-1 (A1) bending
1694 cm-1 (E) stretching
1694 cm-1 (E) bending
3461 cm-1 (A1) stretching
3590 cm-1 (E) stretching
3590 cm-1 (E) stretching
Which mode is highly symmetric?
The mode with frequency of 3461 cm-1
One mode is known as the “umbrella” mode, which one is this?
The mode with frequency of 1090 cm-1
How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
4 bands as the last two frequencies have an intensity of zero.
Charge analysis
As nitrogen is more electronegative than hydrogen, one would expect the N atom to have a more negative charge than the H atoms.
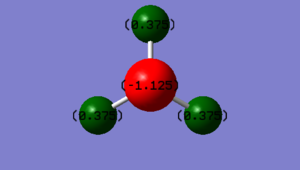
| Atom | Charge | Pauling Electronegativity |
|---|---|---|
| Nitrogen | -1.125 | 3.04 |
| Hydrogen | +0.375 | 2.20 |
N2
| Optimised N-N bond length (Å) | 1.10550 |
| Optimised N-N bond angle (°) | 180 |
| Molecule | N2 |
| Calculation method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy E(RB3LYP) (a.u.) | -109.52412868 |
| RMS gradient (a.u.) | 0.00000060 |
| Point group | C1 (DH) |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Nitrogen molecule |
The optimisation file is linked to here
Vibrational analysis
| Wavenumber (cm-1) | 2457 |
| Symmetry | SGG |
| IR Intensity (KM/mol) | 0 |
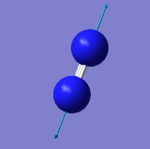
|
Charge analysis
As N2 consists only of atoms of the same element, there is no change in electronegativity. Therefore, the atomic charges are both zero.
H2
| Optimised H-H bond length (Å) | 0.74279 |
| Optimised H-H bond angle (°) | 180 |
| Molecule | H2 |
| Calculation method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy E(RB3LYP) (a.u.) | -1.17853936 |
| RMS gradient (a.u.) | 0.00000017 |
| Point group | C1 (DH) |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Hydrogen molecule |
The optimisation file is linked to here
Vibrational analysis
| Wavenumber (cm-1) | 4466 |
| Symmetry | SGG |
| IR Intensity (KM/mol) | 0 |
|
Charge analysis
As H2 consists only of atoms of the same element, there is no change in electronegativity. Therefore, the atomic charges are both zero.
Comparing H2 with a monometallic TM complex
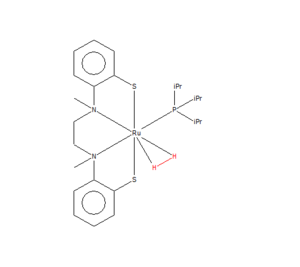 | ||
| [| BIDQUB] | ||
| H2 bond length (Å) | monometallic structure bond length (Å) | |
|---|---|---|
| 0.74279 | 0.912 | |
These bonds are relatively close in length, however the difference could be because the H2 molecule has been bound to the transition metal ligand. The Ru atom is bonded to seven other atoms, leading to a pentagonal bipyramidal shape about it. The bond angles for this shape are 90°, 72° and 180°. These could lead to the H2 molecule is slightly stretched to remain bonded to the central Ru atom. The H-H bond is shorter in the H2, and is therefore stronger than in the ligand.
The Haber-Bosch Process
N2 + 3H2 --> 2NH3
E(NH3) = -56.55776873
2*E(NH3) = -113.1155375
E(N2) = -109.52412868
E(H2) = -1.17853936
3*E(H2) = -3.53561808
ΔE=2*E(NH3) - [E(N2) + 3*E(H2)] = -219.1040481 a.u. = -575257.7 kJ/mol
CO2
| Optimised C-O bond length (Å) | 1.16915 |
| Optimised O-C-O bond angle (°) | 180.00 |
| Molecule | CO2 |
| Calculation method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy E(RB3LYP) (a.u.) | -188.58093945 |
| RMS gradient (a.u.) | 0.00001154 |
| Point group | C1 (Dh) |
Item Value Threshold Converged? Maximum Force 0.000024 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.000021 0.001800 YES RMS Displacement 0.000015 0.001200 YES
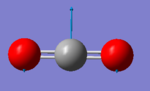
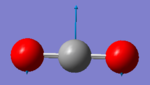
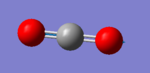
Carbon dioxide molecule |
The optimisation file is linked to here
Vibrational analysis
| Wavenumber (cm-1) | 640 | 640 | 1372 | 2436 |
| Symmetry | PIU | PIU | SGG | SGU |
| IR Intensity (KM/mol) | 31 | 31 | 0 | 546 |
 |
 |
|
Charge analysis
As oxygen is more electronegative than carbon, one would expect the O atoms to have a more negative charge than the C atom.
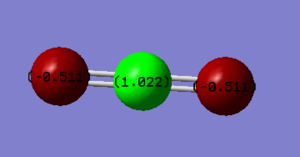
| Atom | Charge | Pauling Electronegativity |
|---|---|---|
| Oxygen | -0.511 | 3.44 |
| Carbon | +1.022 | 2.55 |
Molecular Orbitals (MOs)
Comparing BeH2 to CO2
Both molecules are linear, but BeH2 is made up of much lighter atoms than CO2.
| Final energy (a.u.) | -15.91822472 | -188.58093945 |
| RMS gradient (a.u.) | 0.00008531 | 0.00001154 |
Beryllium dihydride molecule |
The optimisation file is linked to here
Item Value Threshold Converged? Maximum Force 0.000181 0.000450 YES RMS Force 0.000128 0.000300 YES Maximum Displacement 0.001134 0.001800 YES RMS Displacement 0.000802 0.001200 YES
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - you correctly stated two sets of degenerate vibrations. You missed to include this fact when counting the expected number of experimental bands. Taking the degeneracy into account lowers the number of expected vibrations to two. You stated one of the vibrations at 1694 cm-1 to be stretching but actually both of them are bending vibrations.
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES - however, you stated a bond angle for diatomic molecules. To define a bond angle a minimum of 3 atoms is needed!
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
NO - but you included a link to the original paper instead which is fine as well.
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 0/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
NO - you stated the right energies for the products and reactants. The final reaction energy is not correct as it seems you simply summed up the values rather than subtracting the energy of the reactans from the energy of the product.
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
NO - You have not interpreted your result at all.
Your choice of small molecule 3/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES - you could have analysed the calculated vibrations as you did it for NH3 You correctly stated the energies and contributions of AOs to the MOs but you missed to comment on the occupancy and the contribution of the MOs to bonding.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
YES
Do some deeper analysis on your results so far