01381971
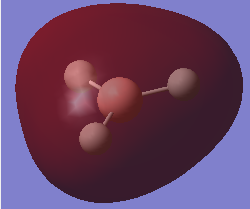
NH3 molecule
information
N-H bond distance=1.01798 Å
H-N-H bond angle=105.741°
calculation method: RB3LYP
basis set: 6-31G(d,p)
final energy E(RB3LYP):-56.44397188 a.u.
RMS Gradient Norm:0.05399560 a.u.
Point Group: C3V
Item Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Jmol Image
test molecule |
Display Vibrations
Questions
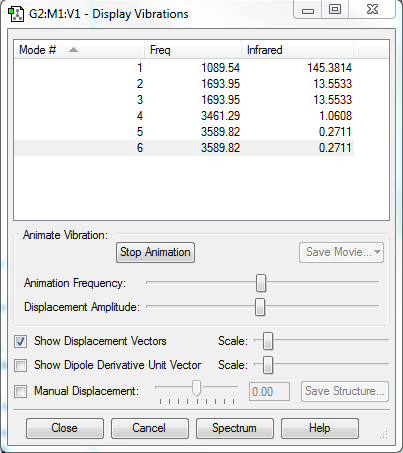
1.How many modes do you expect from the 3N-6 rule?
6
2.Which modes are degenerated (i.e. have the same energy?)
Modes 2,3 and modes 5,6 are degenerated.
3.Which modes are "bending" vibrations and which are "bond stretch" vibrations?
Modes 1,2,3 are bending and modes 4,5,6 are stretching.
4.Which mode is highly symmetric?
Mode 4.
5.One mode is known as the "umbrella" mode, which one is this?
Mode 1.
6.How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
4 bands.
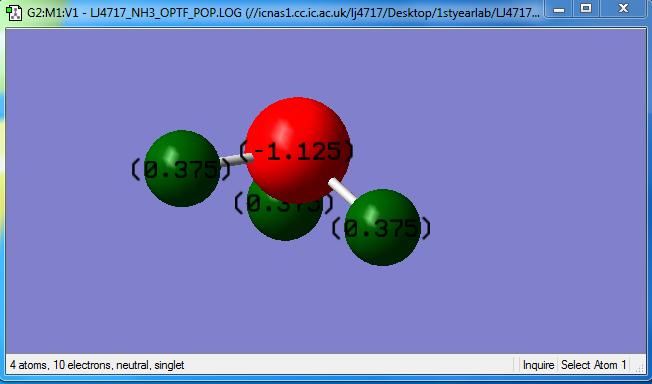
Charges on atoms
charge on N:-1.125 charge on H:+0.375
due to N atom is more electronegative than H atom.
charges
H2 Molecule
General Information
H-H bond distance = 0.74279 Å
H-H bond angle = 180°
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Total Energy: -1.15928020 a.u.
RMS Gradient Norm: 0.09719500
Point Group: D*H
Item
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Jmol 3D Model
H2 Molecule |
Display Vibrations
N2 Molecule
General Information
N-N bond distance = 1.10550 Å
N-N bond angle = 180°
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Total Energy: -109.52359111 a.u.
RMS Gradient Norm: 0.02473091 a.u.
Point Group: D*H
Item
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Jmol 3D Model
N2 Molecule |
Display Vibrations
Energies for Haber-Bosch process
E(NH3) = -56.55776870 a.u.
2*E(NH3)=2*-56.55776870 a.u. = -113.1155370 a.u.
E(N2) = -109.52359111 a.u.
E(H2) = -1.17853936 a.u.
3*E(H2)=3*(-1.17853936 a.u.) = -3.53561808 a.u.
ΔE = 2*E(NH3)-[E(N2)+3*E(H2)] = -113.1155370+113.05920919 = -0.05632781 a.u. = -147.89 kJ/mol As ΔE is negative, the product(nh3) is thermodynamically more stable.
BH3 molecule
general information
B-H bond distance=1.19232 Å
H-B-H bond angle=120°
calculation method: RB3LYP
basis set: 6-31G(d,p)
final energy E(RB3LYP):-26.61511243 a.u.
RMS Gradient Norm: 0.00305259 a.u.
Point Group: D3H
Item
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000017 0.001800 YES RMS Displacement 0.000011 0.001200 YES
Jmol image
BH3 Molecule |
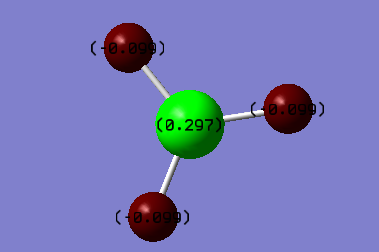
charges
charge on B atom:+0.297
charges on H atoms:-0.099
display vibration
vibration mode 1
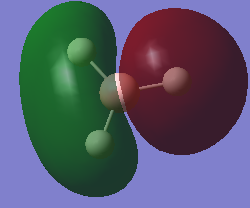
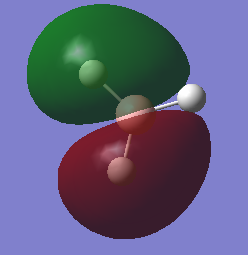
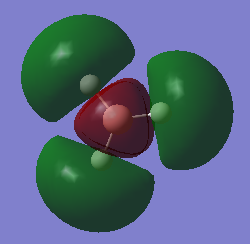
molecular orbital
mo1
mo2
mo3
mo4
mo6