01354725
Ammonia NH3
Detailed Information
N-H Bond Length : 1.01798Å
H-N-H Angle : 105.741°
Calculation Method: RB3LYP
Basis Set : 6-31G(d,p)
Final Energy E(RB3LYP) : -56.55776873a.u.
Point Group : C3V
Item Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Jmol 3D Model
NH3 molecule |
Display Vibrations
Questions
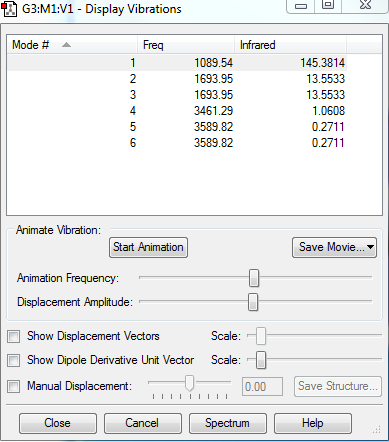
1. How many modes do you expect from the 3N-6 rule?
No. of Modes = 3*4-6 = 6
2.Which modes are degenerate (ie have the same energy)?
The 2nd and 3rd modes are degenerate, and the 5th and 6th modes are degenerate.
3.Which modes are "bending" vibrations and which are "bond stretch" vibrations?
"Bending" vibrations : 1st,2nd and 3rd modes. "Bond Stretch" vibrations : 4th,5th and 6th modes.
4.Which mode is highly symmetric?
5.One mode is known as the "umbrella" mode, which one is this?
6.How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
4 bands.
Charge Analysis
1.Charge on N atom : -1.125.
2.Charge on H atom : 0.375.
3.Overall dipole moment magnitude : 1.8466
The expected N charge is negative and H charge is positive, since the electronegativity of N is 3.04, but that of H is 2.20. N is more electronegative than H. N is more tending to attract a shared pair of electrons (or electron density) towards itself.
N2 Molecule
Detailed Information
N2 bond distance = 1.10550 Å
N2 bond angle = 180°
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy E(RB3LYP): -109.52412868 a.u.
Point Group: D*H
Item Table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Jmol 3D Model
N2 molecule |
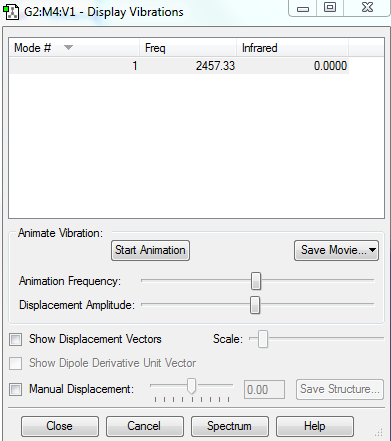
Display Vibrations
H2 Molecule
Detailed Information
N-H Bond Length : 0.74279Å
H-N-H Angle : 180°
Calculation Method: RB3LYP
Basis Set : 6-31G(d,p)
Final Energy E(RB3LYP) : -1.17853936a.u.
Point Group : D*H
Item Table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Jmol 3D Model
H2 molecule |
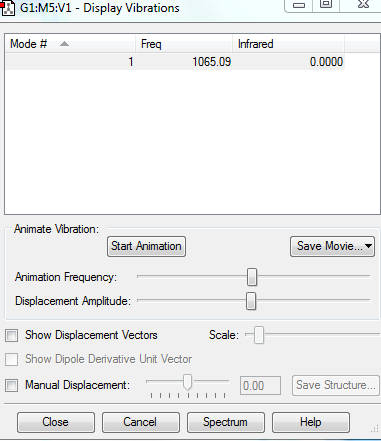
Display Vibrations
Reactivity
E(NH3) = -56.55776870 a.u.
2*E(NH3) = -113.1155370 a.u.
E(N2) = -109.52359111 a.u.
E(H2) = -1.17853936 a.u.
3*E(H2) = -3.53561808 a.u.
ΔE = 2*E(NH3)-[E(N2)+3*E(H2)] = -113.1155370+113.05920919 = -0.05632781 a.u.
= (-0.05632781/ 0.0000038)*0.01 kJ/mol = -147.89 kJ/mol
The ammonia product is more stable because the energy difference is negative,
indicating the reaction forms low energy level product from high energy level product by releasing energy.
F2 Molecule
Detailed Information
F2 bond distance = 1.40281 Å
F2 bond angle = 180°
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy E(RB3LYP) : -199.49825218a.u.
Point Group: D*H
Item Table
Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000128 0.000300 YES Maximum Displacement 0.000156 0.001800 YES RMS Displacement 0.000221 0.001200 YES
Jmol 3D Model
F2 molecule |
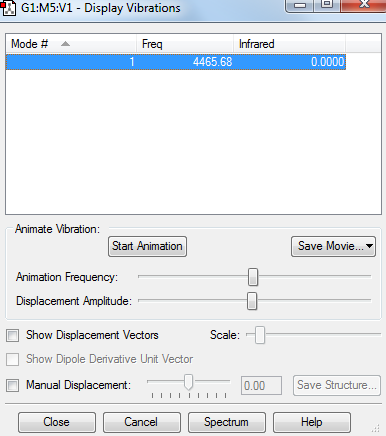
Display Vibrations
Charge Analysis
The charges of 2 F atoms in F2 molecule are same.
The overall charge and dipole moment of F2 molecule are zero.