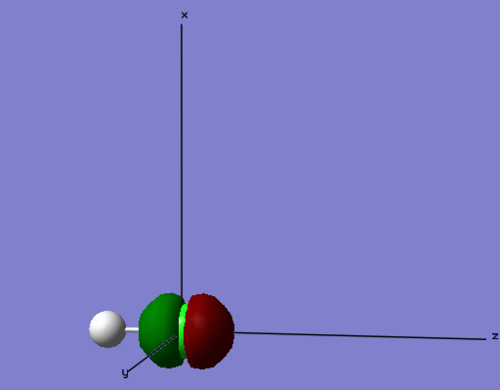
=Mod:67239
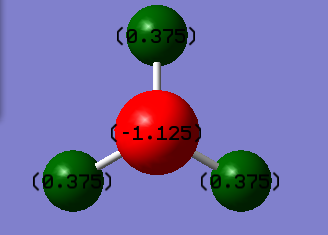
NH3 molecule
NH3 |
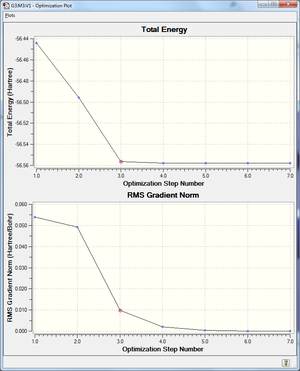
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.164080D-13
Specifications
H-N-H Bond angle - 105.74118
Bond distance - 1.01798Å
Calculation method - RB3LYP
basis set - 6-31G(d.p)
final energy E(RB3LYP): - 56.55 au
Point Group - C3V
RMS gradient - 0.00032440
Atom Chargesː
The file for the molecule is linked here
Molecule Vibrations
FREQUENCY
1 1089.54
2 1693.95
3 1693.95
4 3461.29
5 3589.82
6 3589.82
how many modes do you expect from the 3N-6 rule?
N = 4. 3(4)-6 = 6. There will be 6 vibrational modes for NH3 according to this rule.
which modes are degenerate (ie have the same energy)?
1 and 2, and 4 and 6 have the same energy
which modes are "bending" vibrations and which are "bond stretch" vibrations?
1,2, and 3 represent bending vibrations; 4,5, and 6 are stretch.
which mode is highly symmetric?
4
one mode is known as the "umbrella" mode, which one is this?
1 - the molecule is inverted when it is bent
how many bands would you expect to see in an experimental spectrum of gaseous ammonia?
2 - only two IR active vibrations that result in a change in dipole moment.
Formation of Ammonia
Calculating the Energy Change
E(NH3)= - 56.5576 a.u. 2*E(NH3)= - 113.1152 a.u. E(N2)= -109.52412868 a.u. E(H2)= -1.15928020 a.u. 3*E(H2)= 3.4778406 a.u. ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.1133594 a.u
= -297.6240772kj/mol
identify which is more stable, the gaseous reactants or the ammonia product?
The energy change is negative; the ammonia product has a lower energy than the gaseous reactants and hence is more stable.
confirm we have minimum and not a transition state structure.
The graph shows no negative curvature, therefore there will be no negative second derivative and a maximum will not exist, which would otherwise represent a transition state. The second derivative will hence only have a positive value, representing a minimum.
N2 MOLECULE
N2 |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.401047D-13
Specifications
Bond distance - 1.10550Å
Atom Charges - 0
Bond Angle - 120 degrees
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -109.52412868 a.u.
RMS Gradient Norm = 0.00000060 a.u.
Point Group = D*H
The file for the molecule is linked to here
H2 MOLECULE
H2 |
The file for the molecule is linked to here
Item Value Threshold Converged? Maximum Force 0.000708 0.000450 NO RMS Force 0.000708 0.000300 NO Maximum Displacement 0.000934 0.001800 YES RMS Displacement 0.001321 0.001200 NO Predicted change in Energy=-6.251990D-07
Specifications
Bond distance - 0.600Å
Bond Angle - 120 degrees
Atom charges - 0
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -1.15928020 a.u.
RMS Gradient Norm = 0.09719500 a.u.
Point Group = D*H
HCL MOLECULE
HCL |
Item Value Threshold Converged? Maximum Force 0.000090 0.000450 YES RMS Force 0.000090 0.000300 YES Maximum Displacement 0.000139 0.001800 YES RMS Displacement 0.000197 0.001200 YES Predicted change in Energy=-1.270754D-08 Optimization completed. -- Stationary point found.
Specifications
Bond distance - 1.28599Å
Bond Angle - 120 degrees
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -460.80077875 a.u.
RMS Gradient Norm = 0.00005211 a.u.
Dipole Moment = 1.4334 Debye
Point Group = C*V
Charges:
The file for the molecule is linked to here
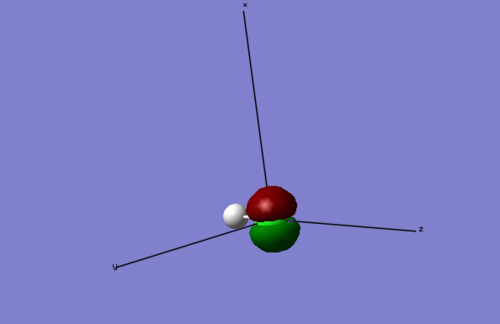
Molecular Orbitals HCʟ
 This shows the unoccupied molecular orbital possessing the lowest energy (LUMO) for the HCL molecule.
This shows the unoccupied molecular orbital possessing the lowest energy (LUMO) for the HCL molecule.
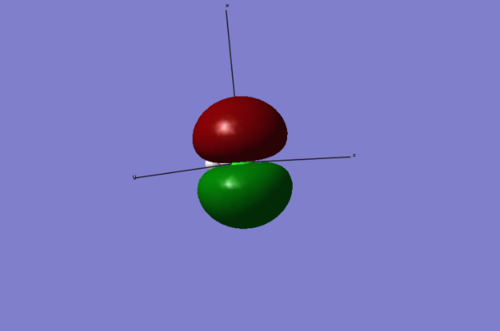 This shows the degenerate 3px HOMO on the chlorine atom. Interestingly, in this case neither of the Highest Occupied Molecular Orbitals are bonding orbitals.
This shows the degenerate 3px HOMO on the chlorine atom. Interestingly, in this case neither of the Highest Occupied Molecular Orbitals are bonding orbitals.
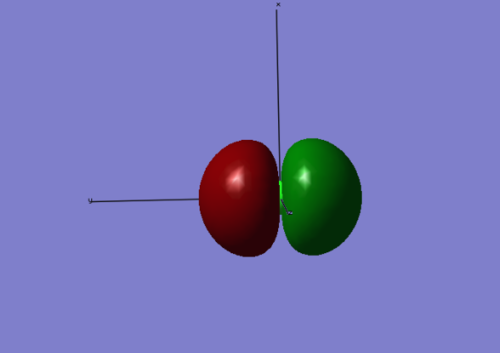 This shows the degenerate 3py HOMO. Again, it is a non-bonding orbital.
This shows the degenerate 3py HOMO. Again, it is a non-bonding orbital.
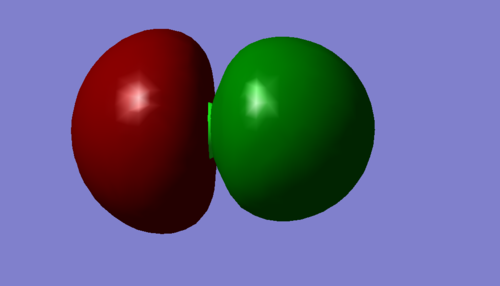
These illustrate further degeneracy in the 3px and 3py orbitals of the clorine atom, illustrated by the directionality of the orbital with respect to the cartesian axis'; both orbitals have the same solution to the schrodinger equation of 7.22836, and hence the same energy.
However, the pz orbital shows a slightly higher solution of 7.23847 despite the atomic orbitals being degenerate for npx, npy, and npz; it is likely interactions with the Hydrogen 1s orbital in the z plane raise the orbitals energy slightly.