User:Xx108 (module 1)
Module 1
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Modelling using Molecular Mechanics
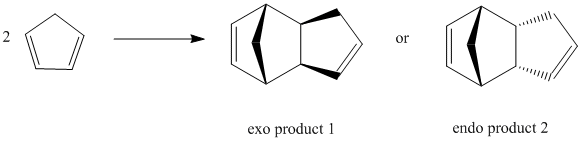
Dimerisation of cyclopentadiene
| stretching / kcalmol-1 | bending / kcalmol-1 | tortion / kcalmol-1 | van der Waals / kcalmol-1 | dipole-dipole / kcalmol-1 | total energy / kcalmol-1 | |
|---|---|---|---|---|---|---|
| exo dimer | 1.2856 | 20.5790 | 7.6571 | 4.2324 | 0.3776 | 31.8766 |
| endo dimer | 1.2507 | 20.8477 | 9.5109 | 4.3194 | 0.4476 | 33.9975 |
According to the above table, the exo dimer has a total energy of 31.8766kcal/mol while the endo dimer has a total energy of 33.9975kcal/mol. This shows that the exo dimer is the thermodynamic product(lower in energy,more stable) whereas the endo dimer is the kinetic product(higher in energy,less stable). For this Diels-Alder dimerisation process, the endo dimer(kinetic product) is formed, which is mainly due to the secondary p orbital interactions[1] between two cyclopentadiene molecules. In addition, the kinetic product(endo dimer)is favoured because it has a lower energy barrier to overcome compared to that for the thermodynamic product(exo dimer).
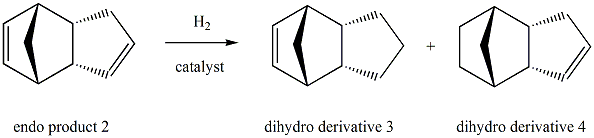
The hydrogenation of cyclopentadiene dimer
| stretching / kcalmol-1 | bending / kcalmol-1 | tortion / kcalmol-1 | van der Waals / kcalmol-1 | dipole-dipole(hydrogen bonding) / kcalmol-1 | total energy / kcalmol-1 | |
|---|---|---|---|---|---|---|
| dihydro derivative 3 | 1.2777 | 19.8661 | 10.8068 | 5.6329 | 0.1621 | 35.6850 |
| dihydro derivative 4 | 1.0958 | 14.5234 | 12.4979 | 4.5123 | 0.1406 | 31.1520 |
The total energy for the dihydro derivative 4(31.1520kcal/mol) is lower compared to that for the dihydro derivative 3(35.6850kcal/mol). This indicates that the dihydro derivative 4 is the thermodynamic product while the dihydro derivative 3 is the kinetic product. As a consequence, formation of product 4 is thermodynamically more favourable and the dihydro derivative 4 is the major product. The difference in total energy(4.53kcal/mol) is mainly accounted for by the difference in bending energy(5.34kcal/mol). This is because the bond angle for product 3(108.20) deviates more from the typical sp2 hybridised carbon framework[2] than that for product 4(112.40), resulting in a larger bending energy for product 3.
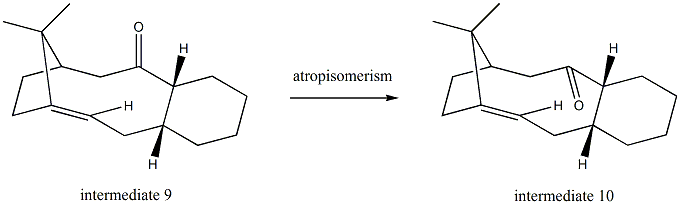
Stereochemistry and reactivity of an intermediate in the synthesis of Taxol
| stretching / kcalmol-1 | bending / kcalmol-1 | tortion / kcalmol-1 | van der Waals / kcalmol-1 | dipole-dipole / kcalmol-1 | total energy / kcalmol-1 | |
|---|---|---|---|---|---|---|
| isomer 9 chair conformer | 2.6837 | 15.8108 | 18.1324 | 12.6057 | 0.1172 | 48.7721 |
| isomer 9 boat conformer | 2.8439 | 16.6597 | 21.4216 | 14.1854 | 0.1480 | 54.9428 |
| isomer 10 chair conformer | 2.5140 | 10.6902 | 19.6076 | 12.5587 | 0.1813 | 44.3379 |
| isomer 10 boat conformer | 2.6533 | 11.1325 | 22.9481 | 13.8626 | 0.1738 | 49.9453 |
For both isomer 9 and isomer 10, the total energy is minimized when the cyclohexane ring is in a chair conformation. Since the chair conformer of isomer 9 has a total energy of 48.7721kcal/mol while the chair conformer of isomer 10 has a total energy of 44.3379kcal/mol, isomer 10 is thermodynamically more stable.
This kind of alkene(hyperstable alkene) is very difficult to undergo hydrogenation simply because it is less strained[3] than the corresponding alkane,which makes it become very unreactive.
| total energy / kcalmol-1 | |
|---|---|
| isomer 9 chair conformer | 70.5536 |
| isomer 10 chair conformer | 60.5774 |
Unlike the MM2 method, the cyclohexane ring is changed automatically to the more stable chair conformation during the minimization process under MMFF94 method. Therefore, only the total energy for the chair conformer of both compounds is shown. The result is consistent with that obtained using MM2. Isomer 10 is again proved to be more stable as its total energy is lower.
Modelling usig semi-empirical molecular orbital theory
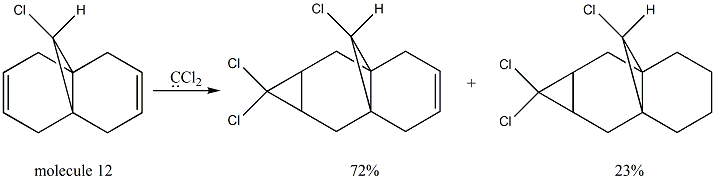
Regioselective addition of dichlorocarbene
Part 1
| LUMO+2 | LUMO+1 | LUMO | HOMO | HOMO-1 |
|---|---|---|---|---|
 |
 |
 |
 |

|
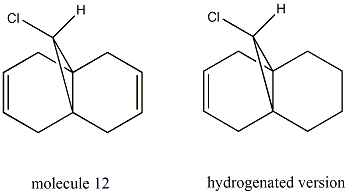
From the MM2 method, the total energy of molecule 12 is minimized to 17.8945kcal/mol. After this, the MOPAC/PM6 method is applied in order to provide a representation of the valence-electron molecular wavefunction. From the pictures above, it can be seen that the electorn density is higher around the syn C=C bond than the anti C=C bond in the HOMO and HOMO-1 orbitals. Therefore, the syn C=C bond is more nucleophilic than the anti C=C bond and is more likely to undergo an electrophilic addition reaction. Since the reaction with dichlorocarbene is analogous to electrophilic addition[4], this method does discriminate between the two alkene bonds. What's more, the molecule 12 itsself has a plane of symmetry but the molecular orbitals shown are unsymmetrical. This is probably due to the limitations of the MOPAC/PM6 method used and it can be improved by introducing an alternative method.
Part 2
| C-Cl stretch | syn C=C stretch | anti C=C stretch | |
|---|---|---|---|
| molecule 12 | 771.82cm-1 | 1756.24cm-1 | 1738.66cm-1 |
| hydrogenated version | 777.82cm-1 | 1759.14cm-1 | - |
From the above table, we can see that the C-Cl stretches for both compounds are in good agreement with the literature[5] (740-780cm-1) and C=C stretches are slightly higher compared to the literature value[6] (1620-1680cm-1). In addition, a larger value od C-Cl stretch is observed for the hydrogenated compound compared to that for molecule 12. After hydrogenation, the interaction between the anti C=C pi orbital and C-Cl sigma* orbital vanishes. In this way, the electrons donated into the anti-bonding orbital of C-Cl bond are fewer, leading to a longer bond length and hence a weaker C-Cl bond.
Monosaccharide chemistry: glycosidation
| stretching / kcalmol-1 | bending / kcalmol-1 | tortion / kcalmol-1 | van der Waals / kcalmol-1 | dipole-dipole / kcalmol-1 | total energy / kcalmol-1 | Heat of formation / kcalmol-1 | |
|---|---|---|---|---|---|---|---|
| A | 2.4331 | 13.9045 | -3.2516 | 17.7430 | 7.8624 | 30.2583 | -78.4023 |
| A' | 2.2607 | 12.5835 | -4.1634 | 17.4102 | 6.5319 | 34.2548 | -73.2374 |
| B | 2.5140 | 13.8565 | -1.5187 | 17.5266 | 7.1408 | 38.2059 | -73.9246 |
| B' | 2.2375 | 11.3798 | -2.2316 | 16.4670 | 6.2301 | 32.3412 | -67.7125 |
| C | 2.0942 | 17.4346 | 3.4250 | 16.1736 | 1.2481 | 33.0317 | -78.7253 |
| C' | 4.2491 | 23.7328 | 5.1734 | 18.0368 | 0.7732 | 47.2654 | -65.1094 |
| D | 2.0112 | 22.4795 | -2.1766 | 14.5730 | 4.9241 | 30.6234 | -74.3058 |
| D' | 2.6781 | 19.8349 | 5.2546 | 17.8437 | 4.0372 | 44.2738 | -61.2453 |
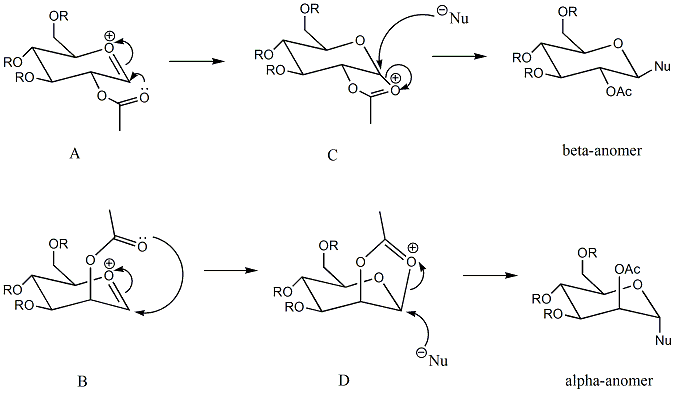
Methyl group is the appropriate R group since it is the smallest one among all the alkyl groups. In this way, the computational demand will be kept at a minimum. Heretwo different methods are used to exmaine the neighbouring-group-participation. Since neighbouring-group-participation is caused by the interaction between the oxygen on -OAc group and the sigma* orbital on C=O+[7] which has a quantum mechanical nature, the MM2 method may not be suitable for this task. Instead, the MOPAC/PM6 method is able to detect this kind of interaction, giving a better result.
As discussed above, MOPAC/PM6 is the more appropriate method. Therefore, when deciding which one of the pairs(A/A' and B/B') has the lower energy, only the results obtained from the MOPAC/PM6 method should be used. The one with a more negative heat of formation value will be more stable.
Similarly the two methods have been used to obtain values for the intermediates C and D as well. According to the heat of formation data, C'/D' is very unstable compared to C/D. In contrast, A'/B' is just slightly unstable comapared to A/B. This shows that C and D predominate in this reaction and it has been approved by literature[8]. As a consequence, it is no longer possible to get two isomers, the reaction is stereospecific.
Structure based mini project using DFT-based molecular orbital methods
Stereoselective dissolving metal reductions
| carbon 1 | carbon 2 | carbon 3 | carbon 4 | carbon 5 | carbon 6 | carbon 7 | carbon 8 | carbon 9 | carbon 10 | carbon 11 | carbon 12 | carbon 13 | carbon 14 | carbon 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| chemical shift calculated/ppm | 74.36 | 42.15 | 40.81 | 33.73 | 41.29 | 30.62 | 24.07 | 38.94 | 25.76 | 38.14 | 33.02 | 150.83 | 105.01 | 23.88 | 15.24 |
| chemical shift from the literature/ppm | 76.83 | 38.82 | 43.35 | 33.48 | 39.93 | 30.95 | 16.88 | 37.31 | 24.57 | 39.19 | 27.35 | 148.25 | 111.76 | 22.49 | 14.72 |
| absolute difference | -2.47 | 3.33 | -2.54 | 0.25 | 1.36 | -0.33 | 7.19 | 1.63 | 1.19 | -1.05 | 5.67 | 2.58 | -6.75 | 1.39 | 0.52 |
| percentage difference | -3.2% | 8.6% | -5.9% | 0.7% | 3.4% | -1.1% | 42.6% | 4.4% | 4.8% | -2.7% | 20.7% | 1.7% | -6.0% | 6.2% | 3.5% |
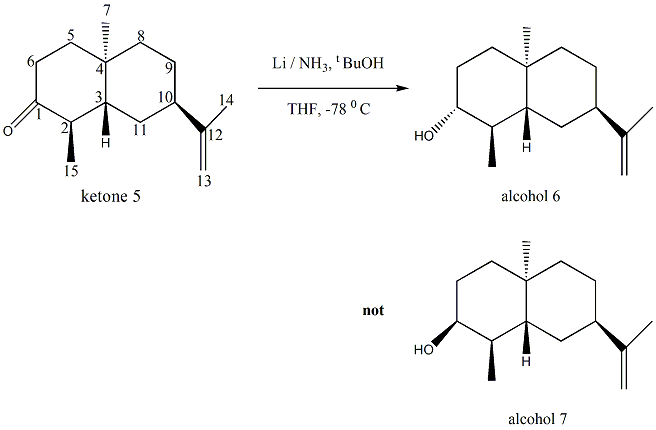
By comparing the IR spectra for both the reactant and the product, it should be able to tell whether the reaction works or not. This is because in ketone 5, there is a strong peak at about 1680cm-1 due to the presence of carbonyl group. In contrast, In either alcohol 6 or alcohol 7, this carbonyl peak will disappear and there will be a broad peak for the -OH group at 3600cm-1. By working out both the sign and magnitude of the optical rotation value, it should be possible to distinguish between the two stereoisomers, namely alcohol 6 and alcohol 7.
From the tabulated results shown above, it can be concluded that the predicted 13C NMR data generally match the reported. The only two exceptions are the values for carbon 7(42.6% difference) and carbon 11(20.7% difference). The others are in good agreement with the literature(all within a 9% difference).
| heat of formation(kcal/mol) | |
|---|---|
| alcohol 6 | -84.45 |
| alcohol 7 | -85.31 |
Alcohol 6 is formed rather than alcohol 7 mainly because of the issue of steric hindrance. When the tBuOH comes towards ketone 5, it will attack from the top face of the molecule because it is sterically less hindered. Despite the fact that the thermodynamic product is usually formed in the dissoving metal reductions of cyclohexanone[9], here the less stable alcohol 6(heat of formation = -84.45kcalmol-1) is formed simply because it is under kinetic control.
References
- ↑ Clayden, Greeves, Warren, Organic Chemistry, 2001, p916-917.
- ↑ J. Sauer, R. Sustmann, Mechanistic Aspects of Diels-Alder Reactions, 1980,19, 780.DOI:10.1002/anie.198007791
- ↑ A.Shultz, L.Flood, J.Springer,Org.Chem., 1986, 51, 838-839.DOI:10.1021/jo00356a016
- ↑ D.Widdowson, The Chemistry of Nitrogen Compounds, Chapter 4
- ↑ G. Socrates, Infrared and Raman Characteristic Group Frequencies, 2001, 65
- ↑ J. Coates, Interpretation of Infrared Spectra, A Practical Approach, John Wiley & Sons Ltd, Chichester, 2000
- ↑ S. Leleu, C. Papamicaël, F. Marsais, Tetrahedron: Asymmetry, 2004, 15, 3919.DOI:10.1016/j.tetasy.2004.11.004
- ↑ ↑ B. Halton, R. Boese and H. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 446.
- ↑ S. Elmore, L Paquette, Tetrahedron, 1991, 32, 3, 318 - 323.DOI:10.1016/S0040-4039(00)92617-0