Wilee5534
Module 1: The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Objectives
In the following exercises a variety of Molecular Mechanics methods are employed to demonstrate how molecular modelling can be used as a tool to determine or confirm the most likely product of a reaction. Molecular Mechanics is the application of classical mechanics to molecules and can be used to obtain the 'optimized geometry' i.e. conformation of lowest energy of a particular molecule alongside a variety of analytical values including bond lengths and Van der Waals interactions.
The Hydrogenation of the Cyclopentadiene Dimer
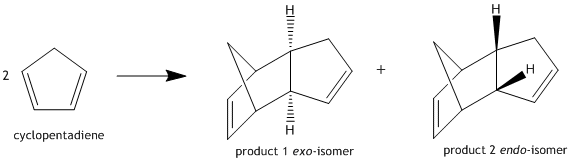
The cyclopentadiene monomer is not stable and undergoes a spontaneous Diels-Alder reaction [4+2 cycloaddition] to form the dimer. This dimerisation is concerted and stereospecific with respect to the dienophile and as such retains its stereochemistry in the product which could be either of 2 isomers [endo or exo] depending on the orientation of attack from the dienophile. Using an MM2 calculation run on ChemBio3D the structures of Species 1 [exo-isomer] and Species 2 [endo-isomer] were minimized and energies recorded. Species 1 has energy 31.8796kcal/mol while Species 2 has energy 34.0136kcal/mol. The results indicate that the exo-isomer is the thermodynamically more stable of the two due to less steric hindrance. However, we know the endo-product is the preferred and this can be seen by taking a closer look at the orbitals of the endo-product.[1]

In the endo product it can be seen that the symmetry is correct for a favourable interaction between the 2 alkenes i.e. endo product is favoured due to favourable orbital interaction across space indicating the reaction is under kinetic control.
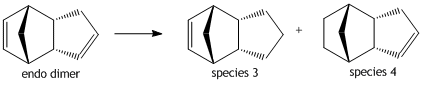
Looking at the hydrogenation of the endo-dimer we see that Species 3 and 4 are the 2 possible outcomes. The 2 species were simulated using ChemBio3D and minimized using the MM2 method in order to compare the ease of hydrogenation of each double bond. Looking at the energies of Species 3 [36.8696 kcal/mol] and Species 4 [29.2543 kcal/mol]; it is expected that hydrogenation would preceed firstly via the double bond of the 6-membered ring followed by the 5-membered ring.
| Species 3[kcal/mol] | Species 4[kcal/mol] | |
| Stretch | 1.2367 | 1.1367 |
| Stretch-Bend | ||
| Torsion | 12.8915 | 12.4291 |
| Non 1,4 VDW | ||
| 4.715 | ||
| 1,4 VDW | 6.0549 | 4.4324 |
| Dipole/Dipole | 0.1632 | 0.1409 |
| Total Energy | 36.8696 | 29.2543 |
From the results of the MM2 Calculation and as already stated Species 4 is the more stable product mainly due to the less torsional strain and smaller bending term. Species 3 has the larger bending term as the H-C-C angles deviates significantly from its ideal 120 degrees [actual = 126.94 degrees]. While Species 4 has an ideal bending term of 109 degrees [actual = 110.3 degrees] and as such deviates much less from the ideal. In reaction to form Species 4 the more strained double bond next to the bridging unit is hydrogenated and as such it is expected that product 4 would be the most stable and therefore is the thermodynamic product while Species 3 is the kinetic product.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
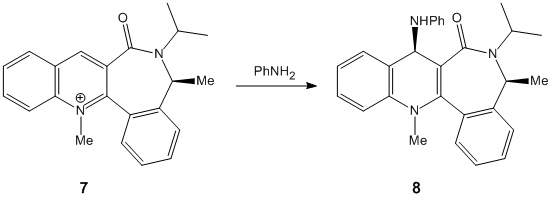
In the following exercise molecular modelling will be used in order to investigate reactions [shown below] of 2 prolinol derivatives [Species 5 & 6]. The first reaction involves the optically active derivative of prolinol[Species 5] and MeMgI while in the second reaction the pyridinium ring of Species 7 is derivatised by reaction with aniline to form Species 8.
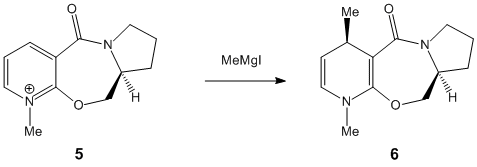
Reaction of Species 5 to form Species 6
The minimum energy found for Species 5 using the MM2 Calculation is 39.1736kcal/mol while the final energy using the MMFF94 minimisation is 57.4888kcal/mol. Another conformer of higher energy [43.1293kcal/mol] of Species 5 is shown. The seven membered ring is quite rigid and it is therefore only the movement of the substituents which have any significant effect on the energy of the molecule but movement of the carbonyl group is restricted. However slight puckering of the 5 membered ring can occur.
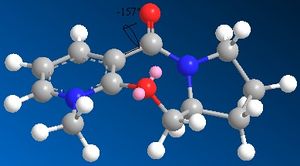
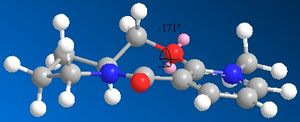
After additon of the Grignard, Species 6 is formed. By varying the geometry of the 5 membered ring a range of conformers of different energies can be found. The absolute stereochemistry of Species 6 can be reasoned by looking at the mechanism of its formation.

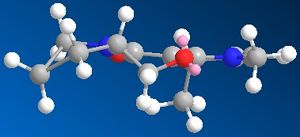
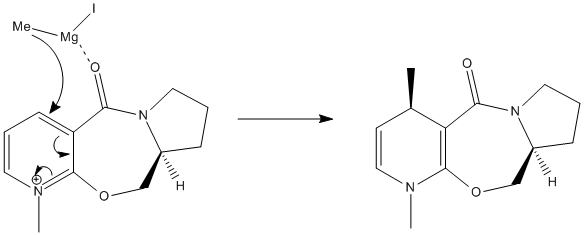
The mechanism has a high degree of regio-stereoselectivity as the incoming MeMgI only attacks at the top face of the ring. The grignard will only add to the 4 position and the reaction proceeds via an electrostatic attraction between the Mg and O as represented by a dashed line in the mechanism above. After several attempts at optimization of Species 6 it can be concluded that the carbonyl group cannot be below the ring and is infact in the plane of the ring. The methyl group must add to the top face of the ring and point upwards. Although regio-stereoselectivity is already high, it could be increased by using a Grignard with a bulkier R group for e.g. -Ph.[2] Unfortunately the Mg component cannot be included in the MM2 Calculations as the calculation is built upon preset parameters for each atom which do not include parameters for Mg.
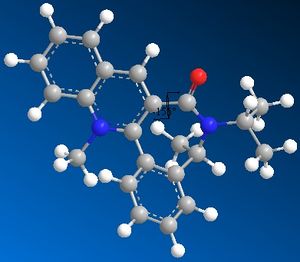
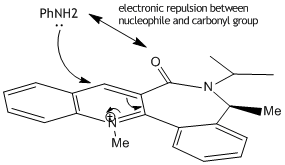
Reaction of Species 7 to form Species 8
As with Species 5 the presence of a seven membered ring restricts any drastic changes in conformation. The minimum energy found using the MM2 optimisation was 62.8933kcal/mol with the carbonyl group slightly below the plane of the ring[~ -20 degrees]. Again the nucleophilic addition to form Species 8 takes place at the 4 position. Looking at the stereocontrol of Species 8; we expect the nucleophile to attack at the opposite side of the ring to the carbonyl group to avoid steric hindrance/electronic repulsion between the O and N and as such product 8 is formed with the NHPh pointing up, away from the ring.
 |
 |
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Structure of the Intermediate
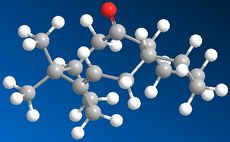
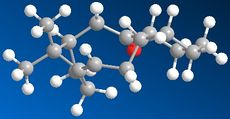
The synthesis of Taxol proceeds via an intermediate which isomerises between Species 9 and 10. Species 9 and 10 are atropisomers which arise from an energy barrier to rotation. Species 9 has the carbonyl group "pointing up" while Species 10 has the carbonyl group "pointing down". Both species were simulated in ChemBio3D and optimized using both the MM2 method and MMFF94 method. With both methods Species 10 was found to be the more stable i.e. with the carbonyl group pointing down.
 |
 |
Species 9
Results of MM2 Calculation: Bend: 15.9089 Stretch-Bend: 0.4011 Torsion: 18.3423 Non-1,4 VDW: -1.2589 1,4 VDW: 12.7359 Dipole/Dipole: 0.1443 Total Energy: 48.9409 kcal/mol
Final Energy using MMFF94: 70.5433 kcal/mol
Species 10
Results of MM2 Calculation Stretch: 3.0633 Bend: 15.0173 Stretch-Bend: 0.3574 Torsion: 21.2414 Non-1,4 VDW: -0.1101 1,4 VDW: 13.6118 Dipole/Dipole: -0.0391 Total Energy: 53.1420 kcal/mol
Final Energy using MMFF94: 74.7832 kcal/mol
Looking more closely at the minimum conformations of Species 9 & 10 it can be seen that the cyclohexane ring must be in a twisted boat conformation for Species 9 and a chair conformation for Species 10. Although significant torsional strain in both species, species 9 has more strain with the carbonyl group pointing up, creating repulsion between adjacent hydrogens on the ring.
Reaction of the Alkene
It has been noted that the alkene present reacts suprisingly slowly. Olefin strain energy [OSE] is defined as the difference between the strain energy of an olefin and the corresponding saturated hydrocarbon.[3] The alkene seen in species 9 & 10 [attached to the bridge head] is unusually unstable and as such the resulting hydrocarbon is less stable than the alkene resulting in a negative OSE.[4]
Modelling Using Semi-empirical Molecular Orbital Theory: Regioselective Addition of Dichlorocarbene
In this exercise a second force field method was introduced [MOPAC/PM6] which uses quantum-mechanical methods in order to include a wave description of the electrons. Using these results the orbital effects and as such the reactivity of Species 12 with dichlorocarbene was investigated.
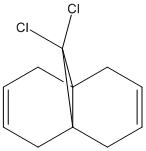
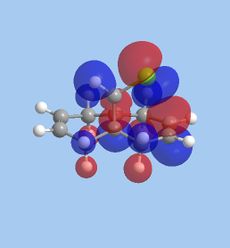
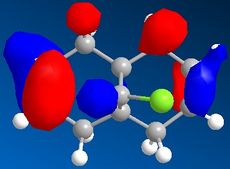
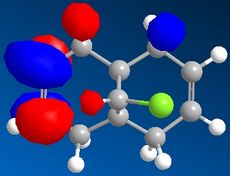
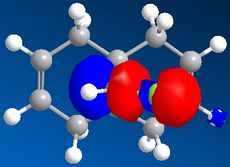
Part 1: Orbital Control of Reactivity
 |
 |
 |
 |
 |
The results of the orbital effect investigation confirm, as expected, that the HOMO is most susceptible to attack from an electrophile. The HOMO distinguishes between the two alkenes; showing greater electron density at the syn-alkene in comparison to the anti-alkene. MM2 minimsation of Species 12 produces a conformation in which the two rings are geometrically distorted. The distance between either of the anti-double bond carbons and the central bridgehead carbon is shorter than the corresponding syn-distance. This is a result of an antiperiplanar stabilisation effect induced by the Cl-C o* (LUMO+1) orbital interacting with the exo-π (HOMO-1) orbital.[5]
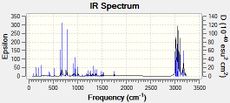
Part 2: Analysing the stretching frequencies
In the following exercise the influence of the C-Cl on the vibrational frequencies of the molecule was investigated. Two species were compared: Species 12 and a hydrogenated version which has the double bond anti to the Cl replaced with a C-C single bond.
 |
 |
C-Cl Stretch: 770.90cm−1
Vibration |
C=C Stretches: anti-alkene; 1737.09cm-1, syn-alkene; 1757.36cm-1
Vibration |
Vibration |
It can be seen that the stretch corresponding to the anti-alkene is lower than that of the syn-alkene suggesting that this double bond has a lower bond energy i.e. weaker bond.
Structure based Mini project using DFT-based Molecular orbital methods
Regio- and stereoselective conversion of alkenes to epoxides
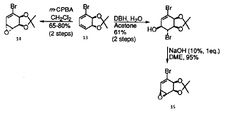
The reaction chosen for investigation involves the regio- and stereoselective conversion of alkenes to epoxides. The reaction [shown below] results in either product 14 or product 15 from the regioselective epoxidation of the 1,3-diene [species 13] depending on which reagents are used. The following investigation demonstrates how molecular mechanics can be used to collect various spectroscopic information for each epoxide allowing comparison with experimental data collected in the lab. In this way, molecular modelling can be used to confirm the identity of the expected product.
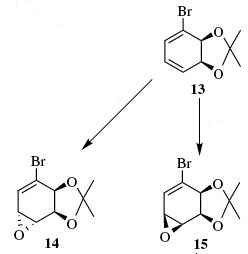
Depending upon the reagents used the reaction yields either species 14 or species 15. Epoxidation of cyclic alkenes is stereoselective, reaction will take place on the less hindered face. As seen in literature if a bulky reagent is used [mCPBA] the epoxide [species14] is formed. This is as a result of steric clash between the dioxolane group present on the molecule. The reaction is forced at the opposite face and as such the newly formed epoxide is "pointing down" while the dioxolane group stays "pointing up" from the plane of the ring. However the reaction may be directed to the more hindered face using the reaction scheme seen in the literature. In other cases, attack on the more hindered face may take place as a result of hydrogen bonding, perhaps if a hydroxyl substituent is present. This is exactly what we see in the synthesis of species 15. In order to direct reaction at the more hindered face; DBH/water is firstly used in order to add a hydroxyl group to the aromatic ring. With hydrogen bonding directing the subsequent attack, reaction at the more hindered face takes place giving rise to species 15 which has the dioxolane and the epoxide on the same face.
 |
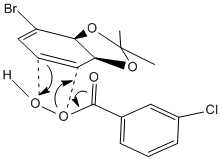 |
Comparison of NMR Data provided by Guassian Calculation with that of Literature
NMR Spectra [13C & 1H] is included below. Each epoxide will have the same NMR data.
13C NMR
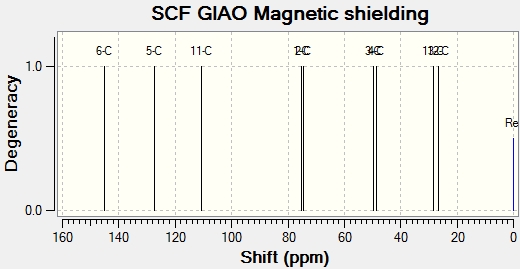
NMR Spectrum for Species 14 & 15 DOI:10042/to-5232 [3aS-(3aalpha,5abeta,6abeta,6balpha)]-4-Bromo-3a,5a,6a,6b-tetrahydro-2,2-dimethyloxireno[e]-1,3-benzodioxole & [3aS-(3aα,5aβ,6aβ,6bα)]-4-Bromo-3a,5a,6a,6b-tetrahydro-2,2-dimethyloxireno[e]-1,3-benzodioxole
13C NMR Data collected from Gaussian Calculation [ppm]: 26.8 (s), 28.3 (s), 48.6 (s), 49.8 (s), 74.3 (s), 75.0 (s), 110.6 (s), 127.4 (s), 144.9 (s).
13C Experimental NMR Data taken from literature [ppm][6]: 25.2 (s), 27.1 (s), 50.2 (s), 54.5 (s), 73.9 (s), 76.8 (s), 108.7 (s), 125.5 (s), 130.0 (s).
The experimental data collected from literature for the 13C NMR is consistent with that produced by the gaussian calculation. The chemical shifts are very similar in each case and demostrate that this particular molecular mechanics method is a reliable way of predicting the NMR of an expected product.
1H NMR
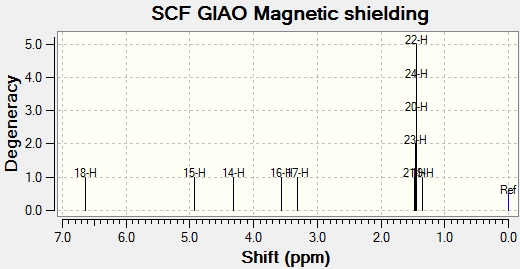
1H NMR Data collected from Gaussian Calculation [ppm]: 1.4 (s, 3H), 1.5 (s, 3H), 3.3 (dd, J = 5.0, 5.0 Hz), 3.6 (m, 1H), 4.3 (dd, J = 6.1, 2.8 Hz, 1H), 4.9 (dd, J = 6.7, 1.9 Hz), 6.6 (d, J = 5.0 Hz, 1H).
1H Experimental NMR Data taken from Literature data[7]: 1.4 (s, 3H), 1.5 (s, 3H), 3.4 (dd, J = 4.5, 4.5 Hz, lH), 3.6 (m, lH), 4.5 (dd, J = 6.6, 2.4 Hz, lH), 4.7 (dd, J = 6.6, 1.8 Hz, lH), 6.7 (d, J = 4.5 Hz, 1H)
Again it can be seen that the NMR data calculated by gaussian has also been reliable for 1H. The calculations appear to have worked especially well for these particular molecules and using Jannochio, accurate 3J-J couplings values have also been found.
IR Spectrum
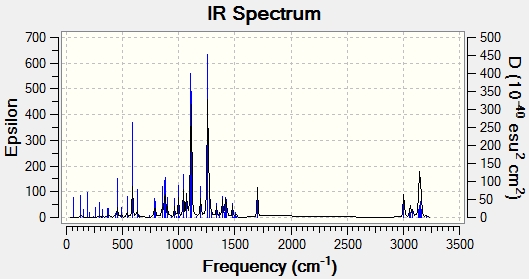
| IR Frequency/cm^-1 | Stretch/Bend |
| 3094 | C-H Stretch Aromatic |
| 2856 | C-H Alkane |
| 1603 | C=C Stretch Aromatic |
| 1267 | C-0 |
| 588 | C-Br |
UV/Vis Spectrum
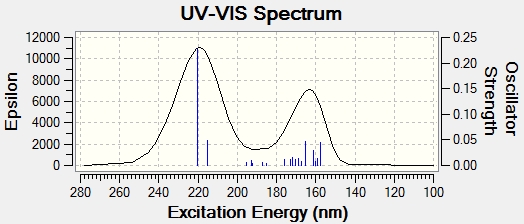
Max wavelength = 220.32nm
Optical Rotation
The results of the Optical Rotation calculation gave a value of -80.1 for species 15 and a value of +83.4 for Species 14. This method is the prinicpal way of distingushing between the isomers given that they will share identical NMR, IR and UV-Vis Spectra. As they are enantiomers, they rotate plane-polarized light by the same amount but in opposite directions and as such the Optical Rotation results agree with that expected. They are also consistent with the value found in literature [-76.2 degrees for species 15].
Conclusion
The previous exercises provide evidence that confirms the usefulness of molecular mechanics/molecular modelling. Starting with a solely classical mechanics method it was shown that the relative energies of various conformations of a particular molecule could be compared in order to predict the moct likely outcome of a reaction. By introducing quantum mechanical methods accurate data [NMR, IR, UV-Vis] was found and has confirmed that these methods are a reliable way of identifying a molecule synthesised experimentally.
References
- ↑ J. Clayden, N. Greeves, S. Warren, P. Wothers; "Organic Chemistry," p.916
- ↑ A.G.Shultz, L.Flood, J.P.Springer, "J.Org.Chemistry," 1986, 51, 838
- ↑ Borden, W. T. Chem. Rev. 1989, 89, 1095
- ↑ "Further calculation explorations and predictions of hyperstable olefins": A.McEwen, P.Schleyer, J.Am.Chem.Soc., 1986, 108(14), 3951-3960
- ↑ Brian Halton and Sarah G. G. Russell J. Org. Chem. 1991,56,pp. 5553-5556
- ↑ Ba V. Nguyen, C. York, T. Hudlicky; "Chemoenzymatic Synthesis of Deoxyfluoroinositols," Tetrahedron, Vol. 53, No. 26, pp. 8807-88141, 1997.
- ↑ Ba V. Nguyen, C. York, T. Hudlicky; "Chemoenzymatic Synthesis of Deoxyfluoroinositols," Tetrahedron, Vol. 53, No. 26, pp. 8807-88141, 1997.
