It:Safrole
| Safrole | ||||
|---|---|---|---|---|
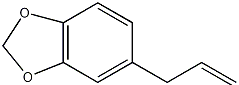
| ||||
| General | ||||
| Systematic name | 5-(2-Propenyl)-1,3-benzodioxole | |||
| Synonyms | Rhyuno oil
Allylcatechol methylene ether | |||
| Molecular formula | C10H10O2 | |||
| SMILES | C12=C(C=C(CC=C)C=C1)OCO2 | |||
| Molar mass | 162.188 g/mol | |||
| Appearance | Colourless to slightly yellow liquid | |||
| CAS number | 94-59-7 | |||
| EC number | 202-345-4 | |||
| Beilstein Registry Number | 136380 | |||
| Properties | ||||
| Density and Phase | 1.095 g/cm3, liquid | |||
| Solubility in water | Insoluble | |||
| Melting point | 11.2°C | |||
| Boiling point | 234.5°C | |||
| Hazards | ||||
| MSDS | Aldrich MSDS | |||
| EU classification | Toxic (T) | |||
| NFPA 704 | ||||
| R-phrases | R45-R22-R68 | |||
| S-phrases | S53-S45 | |||
| Flash point | 97.8 °C | |||
| RTECS number | CY2800000 | |||
| Supplementary data | ||||
| Structure and properties |
n, εr, etc. | |||
| Thermodynamic data |
Phase behaviour Solid, liquid, gas | |||
| Spectral data | UV, FT-IR Condensed Phase, | |||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references] | ||||
Introduction
Safrole is an almost colourless, oily liquid with a particularly pleasant aroma that is common constituent of many essential oils, notably those of the sassafras plant in which it is present to the extent of about 75%, and also as the main component of brown camphor oil. Commonly extracted from the root-bark or fruit of the sassafras plant, it was once commonly used as a food and drink additive - used in the production of root beer and sassafras tea among others - however use was discontinued after animal tests revealed it to be a possible carcinogen.
Safrole is used chemically as a precursor in the synthesis of piperonyl butoxide; an intesticide synergist. It can also be used as a precursor for less savoury products, such as MDMA (more commonly known by its street name of Ecstasy) and MDEA (also known as Eve) - both of which have similar psychedelic and hallucinogenic effects on the human brain. Given its role as a major reactant in certain methods of synthesis of these molecules, safrole is ranked as a List I chemical by the DEA, and also a Table I precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances
Carcinogenic Properties
Studies on animals have shown that, when force fed followed by dietary administration, safrole increased the incidences of liver cell tumors in mice of both sexes. When administered at normal levels in the diet, safrole increased the incidences of liver hepatocellular carcinomas and cholangiocarcinomas in both male and female rats, and hepatocellular carcinomas in male mice. When administered to infant mice by subcutaneous injection, safrole induced lung adenomas and adenocarcinomas in mice of both sexes and hepatomas in male mice.[1]
Further studies showed that this results from metabolites of safrole forming adducts with DNA that induce chromasomal aberrations and sister chromatid exchanges. Such DNA modifications were found to eventually lead to the formation of liver tumours.[2]
However, the absence of evidence on safrole's carcinogenic behaivour in humans has lead safrole to be classified as only category 2b by the IARC.
References
- "Some Naturally Occurring Substances. IARC Monographs on the Evaluation of Carcinogenic
Risk of Chemicals to Humans, vol. 10" International Agency for Research on Cancer, 353 pp.
- Daimon, H., et al., In vivo genotoxicity and DNA adduct levels in the liver of rats treated with safrole. Carcinogenesis 19, 141-146, (1998)

