Organic:Anomeric
The anomeric effect
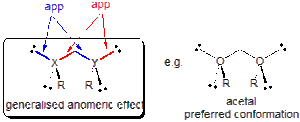
The anomeric effect in its most general form explains the conformational behaviour of systems containing two heteroatoms bound to a single carbon atom i.e. X-C-Y where X and Y are electronegative groups (e.g. acetals, where X = Y = O below):
The anomeric effect was first recognised for the cases of 6-ring acetals (sugars) and is now generally regarded as being mainly stereoelectronic in origin - a stabilisation of a particular conformation as the result of favourable overlap between a filled non-bonding 'donor' orbital (n) that is anti-periplanar to a good 'acceptor' sigma anti-bond (σ*). For example, in organic chemistry the anomeric effect is invoked to explain the following:
The conformation of 6-ring acetals.
These compounds prefer to adopt chair conformations in which the anomeric oxygen is axial. This is in contrast to the situation for cyclohexanes in which the substituent adopts an equatorial position 1) to avoid unfavourable 1,3-diaxial or ‘1,3-flagpole’ interactions, & 2) to minimise gauche interactions:

Two factors favour the α-anomer:
An nOsp3 → σ*C-X anomeric effect which stabilises the α-anomer. The better the σ*C-X orbital is as an acceptor, the stronger the effect:
The α-anomer has a smaller overall dipole moment than the β-anomer:

The conformation of esters.
Esters prefer to adopt s-cis conformations in which all atoms of the group are co-planar:

Co-planarity is stabilised by nOp → π*C=O resonance:
Because the p-orbital on oxygen is symmetrical resonance does not favour either relative to the other. However, there is a relatively strong enthalpic preference for the s-cis conformer over the s-trans one (ΔH° ~25kJmol-1 cf. ~10kJmol-1 for amides) although the barrier to rotation about the acyl oxygen bond (i.e. interconversion) is relatively low ((ΔH# ~50kJmol-1 cf. ~85kJmol-1 for amides). There are three factors which favour the s-cis over the s-trans conformer:
- a) There is a nOsp2 → σ*C-O anomeric effect which stabilises the s-cis form:
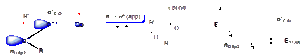
- b) There is significant A1,2 strain in the s-trans form (the sp2 hybrid lone pair on the carbonyl oxygen is ‘small’ relative to a substituent bonded to the acyl carbon atom):

- c) The s-cis form has a significantly smaller overall dipole moment relative to the s-trans form. There is a general preference for conformers with minimum overall dipole (minimum overall charge separation):
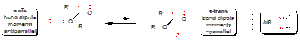
Structural evidence for a stereoelectronic origin to the anomeric effect.
From the 'no bond-double bond' resonance representation the stereoelectronic nOsp2 → σ*C-O interaction shown in (a) above, one would expect that the endocyclic C-O bond should be strengthened/shorter and the exocyclic C-X bond should be weakened/longer than the corresponding bonds for which this interaction is absent. Evidence indicating that this is the case comes from comparison of appropriate crystal structures e.g. bond length analysis of fluoro sugars:

The conformation of fluorocarbonates.
Evidence for this anomeric effect comes from the observation that fluorocarbonates prefer to adopt an s-trans conformation:

Here, the σ* orbital of the C-F bond is a better acceptor than the σ* orbital of the C-O bond (i.e. lower in energy because F is more electronegative than O):
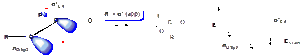
Hence, in these compounds there is a stronger anomeric stabilisation of the s-trans conformation than of the s-cis conformation.
Properties of 5- and 6-ring lactones.
Additionally, 5- & 6-Membered lactones contain an ester function with an enforced s-trans conformation so anomeric nOsp2 → σ*C-O stabilisation is not possible:
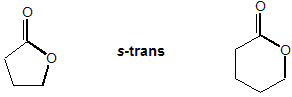
As a result, lactones have some different properties to corresponding acyclic esters:
- a) Lactones are more basic than acyclic esters - because the oxygen sp2 lone pair is ‘more available’ for interaction with protons (e.g. it is possible to form salts etc.)
- b) Lactones are more susceptible to nucleophilic attack at the carbonyl carbon than acyclic esters - because anomeric nOsp2 → σ*C-O stabilisation results in ‘dilution’ of the dipole across the carbonyl in acyclic esters; this interaction is absent for lactones (i.e. they are more electrophilic).
- c) Lactones are also more prone to enolisation than acyclic esters - [pKa ~25 (lactone) cf. pKa ~30 (acyclic ester)] because for acyclic esters there is an energy penalty associated with loss of anomeric stabilisation (nOsp2 → σ*C-O ) in going to the enolate; this is not the case for lactones.
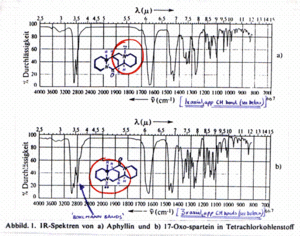
Certain features of the IR spectra of some alkaloids - ‘Bohlmann bands’
Geometrically rigid alkaloids having at least 2 x C-H bonds anti-periplanar to nitrogen lone pairs display characteristic low frequency infra-red stretching frequencies of the C-H bonds.
This is because of multiple nNsp3 → σ*C-H anomeric interactions which weaken the acceptor (i.e. C-H) bonds.
That these bands (2700-2800 cm-1) only occur when there are at least 2 appropriately orientated C-H bonds presumably reflects the weak nature of the interaction [see: E. Winterfeldt Liebigs Ann. Chem. 1994, I-XXXIV (retrospective on Ferdinand Bohlmann 1921-1991)]: